- Development of Orally Absorbable Cisplatin Prodrugs Enclosed in Liposomes and Their Application as a Treatment for Lung Cancer
Jeong Man An# , Kushal Chakraborty*,# , Muhammad Anugrah Pratama*,# , Nipa Banik**, Mi Kwon Son***, and Yong-kyu Lee**, ***,†

Department of Bioengineering, College of Engineering, Hanyang University, Seoul 04763, Korea
*Department of IT and Energy Convergence (BK21 FOUR), Korea National University of Transportation, Chungju 27470, Korea
**Department of Chemical and Biological Engineering, Graduate School, Korea National University of Transportation, Chungju 27469, Korea
***4D Convergence Technology Institute, Korea National University of Transportation, Chungju 7469, Korea- 리포좀에 봉입된 경구 흡수성 시스플라틴 전구약물의 개발 및 폐암 치료제로의 응용
안정만# · 쿠샬차크라보르티*,# · 무함마드아누그라프라타마*,# · 니파바니크** · 손미권*** · 이용규**, ***,†

한양대학교 공과대학 생명공학과, *한국교통대학교 교통에너지융합학과, **한국교통대학교 화공생물공학과, ***한국교통대학교 4D 융합기술연구소
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
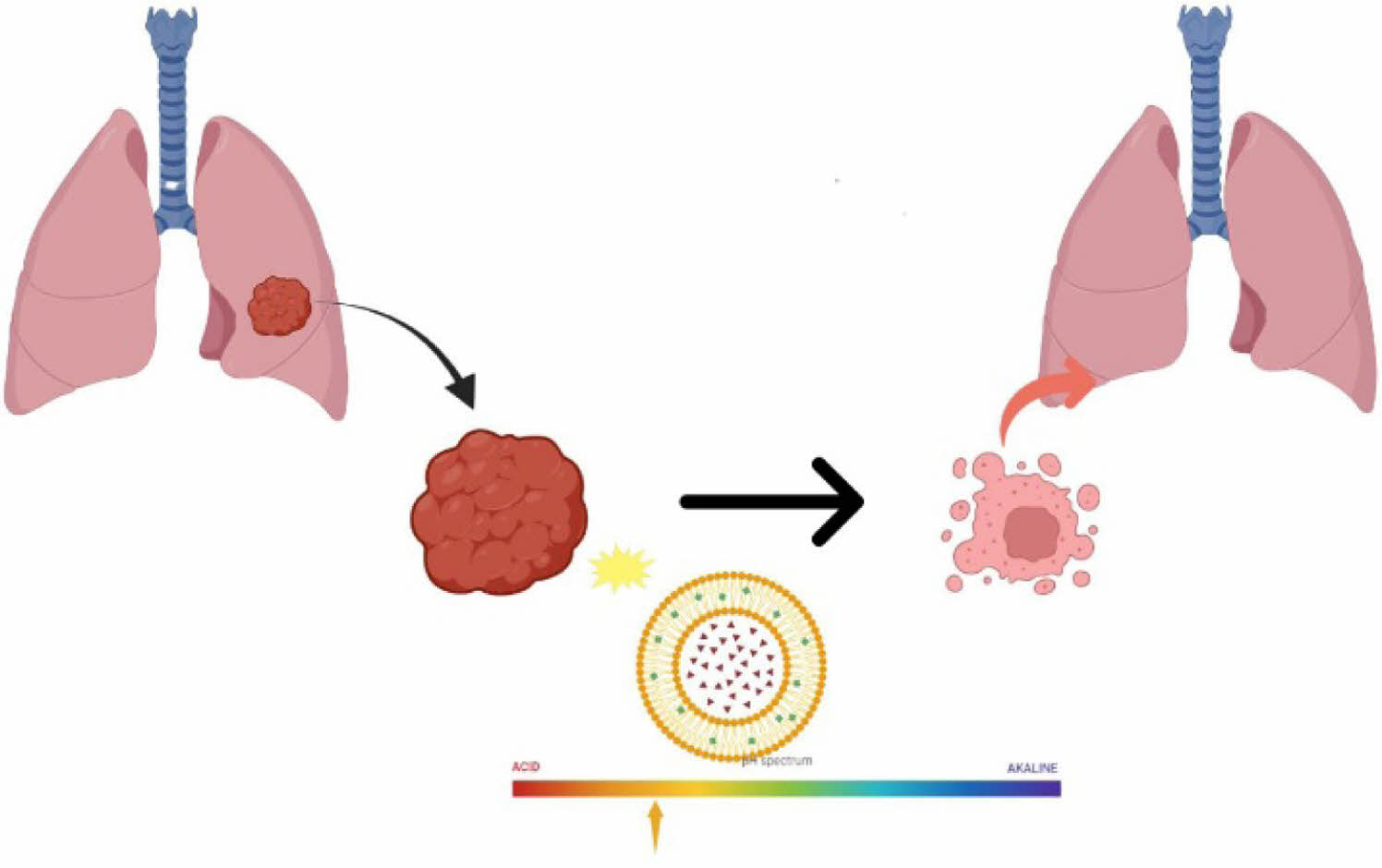
Lung cancer, particularly non-small cell lung cancer (NSCLC), remains a leading cause of cancer-related mortality, with a significant proportion of cases diagnosed at advanced stages. Cisplatin, a cornerstone in cancer therapy, faces challenges such as low water solubility and systemic toxicity. Liposomes have emerged as effective carriers to enhance drug delivery while reducing toxicity; however, the low solubility of cisplatin limits its encapsulation efficiency in the aqueous core of liposomes. This study explores the conjugation of cisplatin with an amine-modified bile acid, sodium taurocholate (TCA-NH2), to enhance its solubility and loading into liposomes. The resulting TCA-cisplatin liposomes exhibited improved encapsulation efficiency, a high drug-to-lipid ratio, and pH-dependent drug release. At the acidic condition (pH 5.5), mimicking the tumor microenvironment, significant cisplatin release was observed, enabling cancer cell-specific delivery. In vitro studies demonstrated effective cytotoxicity against lung cancer cells, comparable to free cisplatin liposomes, with enhanced stability in simulated physiological conditions (pH 6.6). These findings indicate the potential of TCA-cisplatin liposomes as a promising platform for oral administration, paving the way for further development and clinical applications.
폐암, 특히 비소세포폐암(NSCLC)은 암 관련 사망률의 주요 원인으로, 상당수의 사례가 진행 단계에서 진단되는 경우가 많습니다. 암 치료의 초석인 시스플라틴은 낮은 수용성 및 전신 독성과 같은 문제에 직면해 있습니다. 리포솜은 약물의 독성을 줄이면서 전달을 향상시키는 효과적인 운반체로 부상했지만, 시스플라틴의 낮은 용해도는 리포솜의 수성 코어에서 캡슐화 효율을 제한합니다. 이 연구에서는 시스플라틴을 아민으로 개질된 담즙산인 타우로콜산나트륨(TCA-NH2)과 접합하여 용해도와 리포솜 내 로딩을 향상시키는 방법을 탐구합니다. 그 결과 TCA-시스플라틴 리포솜은 향상된 캡슐화 효율, 높은 약물 대 지질 비율, pH 의존적 약물 방출을 보였습니다. 종양 미세 환경을 모방한 산성 조건(pH 5.5)에서 상당한 시스플라틴 방출이 관찰되어 암세포에 특이적으로 전달할 수 있었습니다. 시험관 내 연구에서는 폐암 세포에 대해 유리 시스플라틴 리포솜에 필적하는 효과적인 세포 독성이 입증되었으며, 생리적 조건(pH 6.6)에서 안정성이 증가되었습니다. 이러한 결과는 경구 투여를 위한 유망한 플랫폼으로서 TCA-시스플라틴 리포솜의 잠재력을 보여주며, 향후 개발 및 임상 적용을 위한 길을 열어줍니다.
This study addresses the limitations of cisplatin in lung cancer therapy by conjugating it with amine-modified sodium taurocholate (TCA-NH2), significantly improving its solubility and encapsulation efficiency in liposomes. The resulting TCA-cisplatin liposomes demonstrated enhanced drug loading, pH-responsive release, and effective cytotoxicity against lung cancer cells, suggesting strong potential for targeted and stable drug delivery in clinical settings.
Keywords: cisplatin prodrug, drug delivery, liposome, bile acid, lung cancer.
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (Grant No. 2021R1A6A1A03046418). Additional support was provided by the NRF, funded by the Ministry of Science and ICT (MSIT) of the Korean government (Grant Nos. RS-2024-00405287 and 2021R1A2C2095113).
The authors declare that they have no competing interests.
Information is available regarding the characterization for cisplatin, TCA-NH2, TCA-cisplatin. The materials are available via the internet at http://journal.polymer-korea.or.kr.
PK_2025_049_05_570_Supporting_Information.pdf (660 kb)
Supplementary Information
Among other types of cancer, lung cancer is the most common occurrence and is often diagnosed in the late stages.1 Even with the highest mortality rate, 20~25% of patients with non-small cell lung cancer (NSCLC) undergo surgery and 30~55% can be treated with radical surgery. Although 16% survive up to 5 years after treatment, but patients often die.2 Treatment is improving day by day, but the prognosis is poor.
Several carriers have been developed to deliver cisplatin, reducing its toxicity and improving its anticancer properties. As an alternative carrier, liposomes provided a method for delivering drugs while minimizing toxicities. However, cisplatin's low solubility in water (2 mg/mL) again prevents its loading in the aqueous portion of the liposome. Consequently, it is necessary to conjugate the drug with a hydrophilic complex to improve its water solubility.3,4
From the beginning of the formulation process, different materials are added to the drug to increase its loading,5,6 prolong its release,7,8 or reduce its toxicities.8 Previously, it has been disclosed that a coordination complex can be synthesized by ligand exchange using equated cisplatin and aminopropyltriethoxysilane (APTES) with the amino end linked directly to platinum. Nanoparticles containing silica-cisplatin prodrug conjugate have amino linkers that decrease metabolic activity and increase membrane permeability. In a study of breast cancer cells undergoing chemotherapy, cisplatin was released using the amino end of silica as a carrier with high drug loading, acid-responsive release, and effective cytotoxicity.9
Although oral delivery is the most straightforward method of administering drugs, it has a high acid content in the stomach, low absorption through epithelial membranes, or breakdown of the chemical structure of the drug at physiological pH.10 The conjugation of a solubilizer can improve the drug under such circumstances and overcome the physiological pH. Various studies have shown that bile acid conjugated to the drug allows it to be absorbed more rapidly by the bile acid transporter in the intestine.11 It is possible to facilitate the solubility of drugs by conjugating them with hydrophilic bile acids or their derivatives.12 An amine-modified sodium taurocholate (TCA-NH2) was used in this study to enhance the cisplatin loading in liposomes and the in-vitro anticancer activity.
Materials. Cisplatin (³99.9%), 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPC 98.50%) and D-alpha tocopherol succinate (VES) were purchased from Sigma-Aldrich (USA). DMG-PEG (2k) was purchased from TCI chemicals and ACROS ORGANICS. Taurocholic acid (TCA ³95.0%, Mw: 537.69 g/mol), 4-nitrophenyl chloroformate (4-NPC), Dimethylaminopyridine (DMAP) and o-phenylenediamine (OPDA) were purchased from Sigma-Aldrich (USA). Triethylamine (TEA), ethylenediamine (EDA), ethyl acetate, dichloromethane, diethyl ether, dimethylformamide (99.8%) and acetone were purchased from Sigma Aldrich. Slide-A-Lyzer TM dialysis cassette (3.5 K MWCO) of 3 mL and Amicon Ultra -4 centrifugal filter unit 4 mL was purchased from Merck Millipore. The Dialysis Tubing MWCO 100-500 D was purchased from Spectrum Laboratories (USA).
Cell Culture. From Cellgro (Manassas, VA) Dulbecco’s Modified Eagle Medium (DMEME), Dulbecco's phosphate buffered saline (DPBS), fetal bovine serum (FBS), 0.25% trypsin/EDTA solutions and from Mediatech (Manassas, VA) Penicillin-streptomycin solutions were obtained; those used in this experiment. From American Type Culture Collection (ATCC, Manassas. VA, USA) lung carcinoma epithelial cell line (A549) and epithelial cells of kidney tissues (MDCK) were purchased. 10% FBS and 1% penicillin-streptomycin were used to supplement DMEM, which was the media to culture A549 and MDCK cells. In this media all the cell-culture experiments were performed with the help of an incubator set at 37 ℃ and 5% CO2 atmosphere.
Synthesis of TCA-conjugated Cisplatin Prodrug (TCA-Cisplatin). TCA-NH2 was synthesized by following the previous method with modification.13 In the beginning TCA was activated by conjugation with 4-NPC to form TCA-NPC. TCA-NPC was combined with EDA in presence of TEA to finally synthesize the reactive form of TCA salt to amine form as TCA-NH2. One mol of cisplatin was suspended in approximately 5 mL of distilled water for 1 hour to maintain enough conversion of cisplatin to mono-hydroxy cisplatin. 1.2 mol of TCA-NH2 dissolving in distilled water was added dropwise with sparingly stirring the cisplatin solution to proceed the reaction. After 24 hours the reaction mixture was centrifuged, and the collected supernatant portion was filtered through a 0.22 µm syringe filter. The resultant filtrate solution was dialyzed by 100~500 Da (Biotech grade cellulose ester membranes) for 3~4 days until the brown colour of TCA-NH2 disappears. Finally, the dialyzed cisplatin-TCA solution was freeze dried. Similarly, cisplatin was conjugated with TCA-NH2 in 1:0.5, 1:1, 1:2, and 1:5 ratios respectively to compare the yield, purity, and the toxicity of cells.
Fourier transform infrared (FTIR) spectroscopy of cisplatin, TCA-amine and cisplatin-TCA were performed by the Nicolet 5700 instrument (Nicolet Instrument, Thermo Company, USA) with KBr pellet method. The scanning of each KBr disk was 400-4,000 cm-1 and the resolution was 4.0×108 cm. 1H NMR spectra of the synthesized compounds were recorded with a Bruker DRX 400.
As the reaction media was water there can be three types of conjugate formation (I, II and III) (Scheme S1). Each conjugate has different acid dissociation constant (pKa) values that indicate how many TCA-NH2 units were conjugated.14
Preparation of TCA-Cisplatin Prodrug-nanoparticles Loaded Liposomes (L-TCA-cisplatin). TCA-conjugated cisplatin prodrug-loaded nanoparticles liposome formulations were done through the microfluidic system; by mixing the organic (lipid solution) phase and the aqueous phase at a particular ratio. PreciGenome's NanoGenerator TM applied microfluidic (NanoGenerator Flex-M) equipped with a micro mixture that was a microchip used for the preparation of L-CisTCA. 5 mg of TCA-Cisplatin was dissolved in 10 mL PBS and this aqueous phase was injected to the right inlet of the microchip. Then the organic phase containing DOPC, DMG-PEG(2k) and VES (45:0.2:1) was prepared, under the condition of drug-to-total lipid ratio (D/L) 1:5 dissolving in 2 mL of ethanol; was injected to the left inlet with a total flow rate (TFR) 6 mL/min and volumetric flow rate ratio (FRR) 1:5 (mM). Both the phase: organic and aqueous solutions were filtered through a 0.22 µm syringe filter before injecting in the microfluidics machine. The resulting dispersions of L-CisTCA were collected through the outlet of the microchip and kept at 4 ℃ to reach equilibrium for several hours. The collected liposome was dialyzed at room temperature with a dialysis cassette (MWCO 3.5 kDa) in PBS overnight to remove unloaded drug or free drug. Then L-CisTCA was kept at 4 ℃ for further analysis.
The particle size, zeta potential, PDI and encapsulation efficiency were measured by DLS (Malvern company, model: Zetasizer Nano-ZS90) and UV-visible.
HPLC Condition. Thermo Fisher Scientific HPLC system (UltiMateTM 3000 series) was regulated with chromeleon 7 software and was equipped with an injection valve (quaternary). To separate the synthesized conjugate prodrug from unreacted cisplatin, a reversed-phase C18 column (Zorbax Eclipse Plus C18, 4.6 mm ID 250 mm L, porosity size 5 μm) was set inside the HPLC system. For Tca-cisplatin conjugates the UV absorbance was 305 nm at 37 ℃ with mobile phase A (Water): mobile phase B (Methanol) = 7:93 (v/v) respectively; that was adjusted pH 2.5 by methane sulphonic acid. It followed an isocratic elution with flow rate 0.5 mL/min along 10 min retention time.15
Encapsulation Efficiency and Drug Loading. The encapsulation efficiency of TCA-cisplatin@liposome was determined through derivatizing cisplatin with o-phenylenediamine (OPDA) via UV-visible spectrophotometric method.16 The liposome was heated at 90 ℃ for 10-30 minutes in DMF with 600 times more concentrated OPDA than concentration of cisplatin present in the liposome. The ratio of DMF: water was 7:3 (v/v) and the pH was changed to 6.2 by adding 0.1N HCl. The UV-absorbance of the derivatizing product was taken at 706 nm on a UV-visible multi-mode reader (Agilent BioTek Synergy HTX). The cisplatin entrapped in the liposomes were determined from the standard calibration plot. By the help of the following equations the percentage of drug entrapment efficiency (% DE) and drug loaded (DL) in per mg of lipids were determined.

where, WE, WT, and WP were encapsulated cisplatin in liposomes, total cisplatin, and total lipids respectively.
In Vitro Drug Release of TCA-Cisplatin@liposomes. In vitro drug release of TCA-Cisplatin loaded liposomes were done through the dialysis method at various pH values. 200 µL of liposomes was diluted up to 1 mL with addition of distilled water and transferred to a 3.5 kD dialysis bag. This dialysis bag was placed in 100 mL of different pH values of PBS for 25 hours at 37 ℃. For pH 5.5 sodium acetate buffer was prepared and release study was done in it. The stirring was at a fixed rpm and temperature at 37 ℃, after a specific time interval 500 µL aliquots of PBS medium were withdrawn and stored at 2-8 ℃. Same amount of fresh medium was added to the dialysis medium. Again, the release of cisplatin from liposome was determined by spectrophotometric method via UV-visible multi-mode reader after OPDA bind with cisplatin as a derivatized product.
The equation was used to figure out the percentage of drug release described as below:
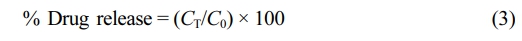
where, CT and C0 are respectively the cisplatin amount released at t time and the total amount of cisplatin in the liposomal dispersion.
Characterization of TCA-conjugated Cisplatin Prodrug (TCA-Cisplatin). Cisplatin was conjugated through equatorial bond with -NH- linkage of TCA-NH2 (Scheme 1), directly with Pt in water medium. As cisplatin has very poor solubility in water after reaction there are some unreacted cisplatin remaining at the bottom of the reaction vial. To avoid this precipitation after dissolving cisplatin in water, the medium was heated at 40-60 ℃ for a few minutes. Then the reaction mixture was centrifuged and collected the supernatant to react with TCA-NH2. Ultimately, the TCA-Cisplatin prodrug was purified through dialysis (100-500 Da) with a yield of 70%. The Scheme 1 signifies step by step the synthesis protocol of TCA-cisplatin elaborately.
The number of TCA-NH2 units conjugated to cisplatin were determined through calculation of the acid dissociation constant (pKa) value. During the reaction (after 24 hours), trace amount of reaction mixture was titrated with 0.5 M NaOH and pKa was calculated. This process was run 2 to 3 times and the pKa was 6.1 ± 0.01. According to the pKa value it was found that at a physiological pH the reaction mixture resulted mostly in the form of the mono-aqua-cisplatin complex I (Scheme S1). As there was a possibility to have complex II and complex II (Scheme 2) with TCA-cisplatin conjugate, so the acid dissociation constant was calculated to confirm which complex was mostly present. The pKa value was determined at different times (24 hours and 48 hours) of a reaction to minimize the presence of a mixture of different final products (I, II and III). The titration was done through titration with NaOH to neutralize the H+ released from hydroxy cisplatin. After adjusting pH like this there was an equivalence point where the pH and the pKa were equivalent. That is the pKa of the reaction mixture and for TCA-cisplatin it was 6.1±0.01 that confirmed the mono aqua-cisplatin was mostly reacted with TCA-NH2 to form TCA-cisplatin (I) complex (Scheme 2).14 For diaqua-cisplatin formation the pKa should be initially 5.5 and finally 7.3 of the reaction mixture.17
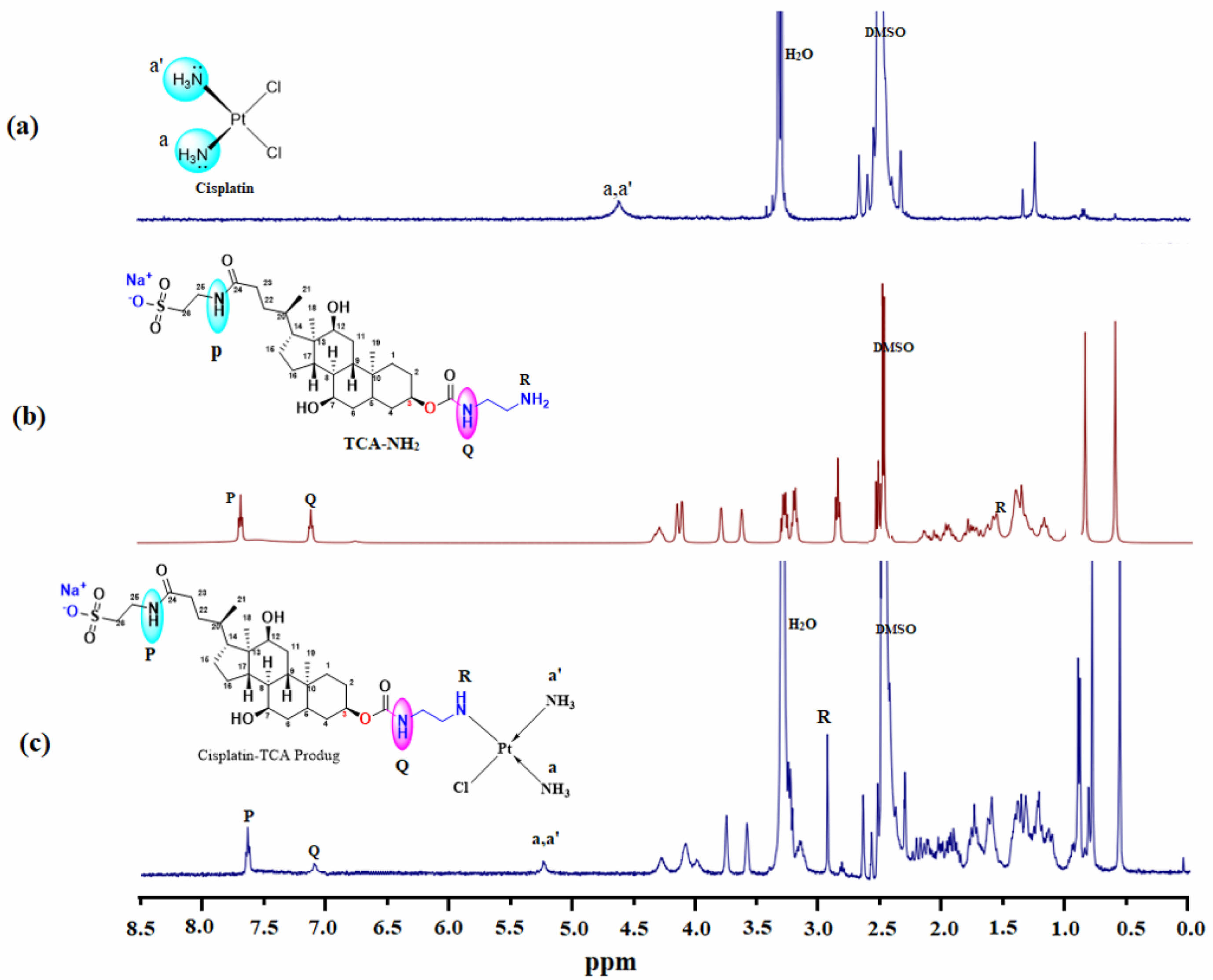
The successful conjugation of cisplatin with TCA-NH2 was confirmed by 1H NMR. Figure 1(c) represented peak at 5.5 ppm that signified the presence of -NH3 from cisplatin, whereas peaks from the range of 0.5-4.3 ppm are indicating TCA-NH2 moiety.18 At 3.3 ppm in Figure 1(a) there was a moisture peak that was observed in the final product also. There are three amine peaks in TCA-NH2: two of them are secondary (P, Q) and one is primary amine (R) in Figure 1(b). The secondary amines were at 7.5 and 7.0 ppm and the primary amine was de-shielded towards 2.9 ppm from 1.5 ppm that indicates the successful conjugation. The primary amine has been converted to secondary amine in the final product TCA-cisplatin.
After the synthesis of TCA-cisplatin pro-drug the conjugate represented whitish brown colour compared to the yellowish cisplatin. At (cisplatin: TCA-NH2) ratio of 1:0.5 (Figure S1(a)) unreacted cisplatin precipitation (yellow) was observed but increasing ratio towards 1:1, 1:2 and 1:5 (Figure S1(b)-(d) respectively) the precipitates disappeared. Increasing the molar ratio of TCA-NH2 0.5, 1, 2 and 5 while fixing a constant amount of cisplatin; TCA-moiety was observed rising in Figure S1. So, there was excess TCA-NH2 in (cisplatin: TCA-NH2) of (1:2) and (1:5).
A Fourier-transform infrared spectrum (FTIR) was used to analyse the chemical structure of cisplatin, TCA-NH2 and cisplatin-TCA prodrug. FTIR results demonstrated the symmetric amine bending modes at 1531.20 cm−1 and 1644.01cm−1 and the characteristic amine stretching mode at 3291.1 cm−1 and 3487.2 cm−1 frequency regions appeared (Figure S2(a)). Platinum and ammonium bond emerged in 481.15 cm−1 and platinum and chloride in 336.41 cm−1 and 365.11 cm−1. Additionally, the out of plane frequency for N-H bond appears at 809.95 cm−1.19-22 In case of TCA-NH2, the frequencies at 1148.12sym.cm−1 and 1214.93asym. cm−1 correspond to S=O group and 1540.84 cm−1 for N-H bending and 1652.69 cm−1 correspond to C=O str. The overlapped frequencies for primary amine, secondary amide and hydroxy groups appeared at approximately 3200-3650 cm−1 (Figure S2(b)). However, the str. frequencies for secondary amide and hydroxy group appeared separately at 3319.85 cm−1 and 3619.73 cm−1, respectively (Figure S2(c)). Notably, the disappearance of Pt-Cl frequencies in the 365.11 cm−1 and appearance of the str. frequencies of S=O groups at 1208.18 cm−1 and 1192.47 cm−1 confirm the formation of Cisplatin-TCA prodrug (Figure S2).
HPLC Condition. The purity of TCA-cisplatin was confirmed by HPLC and sample preparation for raw cisplatin was done in 0.9% NaCl solution that keeps cisplatin without being aquated in the solution up to one hour.15 The purity of TCA-cisplatin conjugate was 98% with no presence of free cisplatin.
Moreover the high resolution mass spectroscopy (HR-MS) of the conjugate was characterized and m/z: for C29H55ClN5NaO8PtSNa+ [M+Na]+ : calculated = 887.37, observed = 803.37.
PEGylation of the TCA-cisplatin Prodrug Nanoassembly and Surface Decoration with Amphiphilic Materials After Formation of Liposome. An amphiphilic polymer, 1,2-Dimyristoyl-sn-glycero-3-methoxypolyethylene glycol (DMG-PEG 2k) was used to improve the surface of the prodrug through PEGylation and that would develop the drug (cisplatin) absorption by increasing solubility. An additional advantage of DMG-PEG 2k is, it prohibits phagocytosis and prolongs the time of the blood circulation of nanoparticles.23
To maintain high drug loading as a result of nanoassemblies, a molar ratio of (5:1) was maintained in between the TCA-cisplatin prodrug and DMG-PEG respectively. Another lipid resembling cell membrane was used named 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPC) with D-alpha tocopherol succinate (VES) to facilitate the stability of nano assembled liposome in a molar ratio of (45:25) respectively. With the help of a microfluidics machine, following the above protocol the TCA-Cisplatin prodrug-nanoparticles loaded liposome (L-CisTCA) was successfully prepared without any precipitation. A liposomal composition for Cisplatin was also prepared with same lipids, same molar ratio to form similar drug loaded liposome. Again, a blank liposome was prepared to compare with TCA-cisplatin@liposome.
The entrapment efficiencies and vesicular size were methodically listed for different liposomes presented in Table 1 and 2. The entrapment efficiency of L-CisTCA was 94% near to L-Cis that was 97% (Table 1); although 0.34 mg and 0.12 mg of cisplatin was loaded per mg of lipid in L-CisTCA and L-Cis respectively.
The vesicular size of the TCA-cisplatin nano particles loaded liposome (L-CisTCA) were found in nano scale size and with a negative zeta potential (Table 2). As the nano size of the liposome determines the efficacy of a loaded chemotherapeutic, it is a vital factor. It affects the cellular uptake, cellular transfection efficiency and intracellular concentration of the drug.24 As the L-CisTCA liposome represents the particle size of 146.7 ± 1.0 nm, thus it has the capacity to enter the targeted cells.25 The zeta potential of free cisplatin loaded liposome and cisplatin conjugated TCA pro-drug nano particles loaded liposome were respectively -1.28 mV and -13.7 mV; with a poly-dispersity index (PDI) 0.04 and 0.6 respectively.
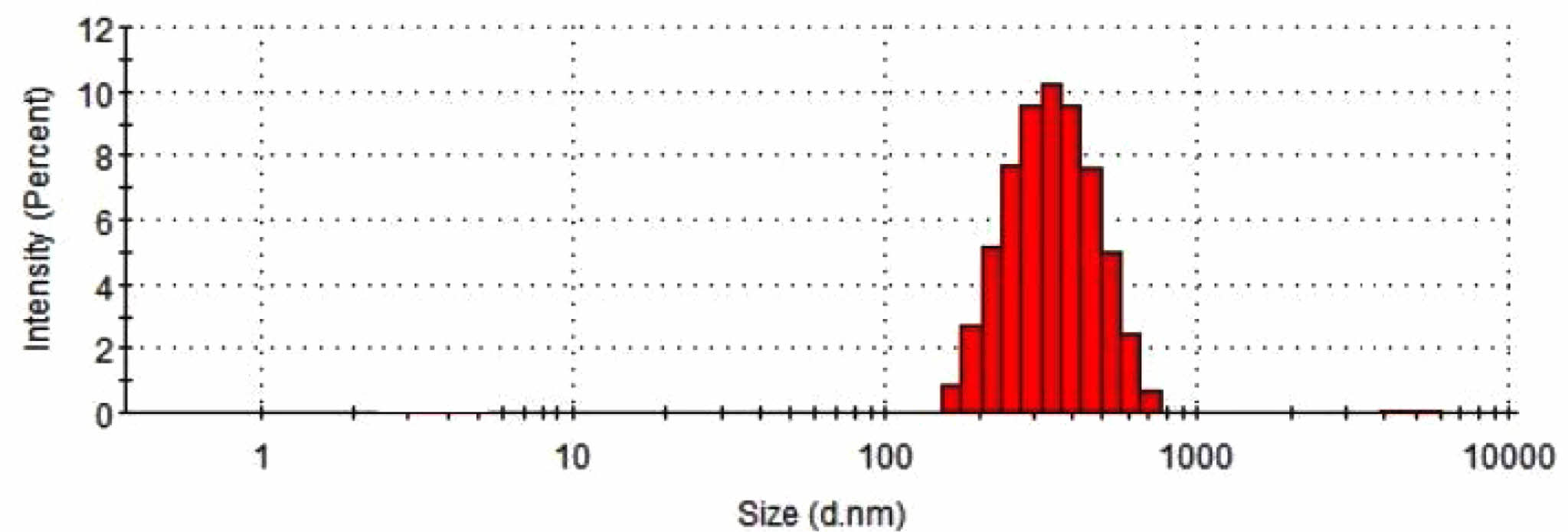
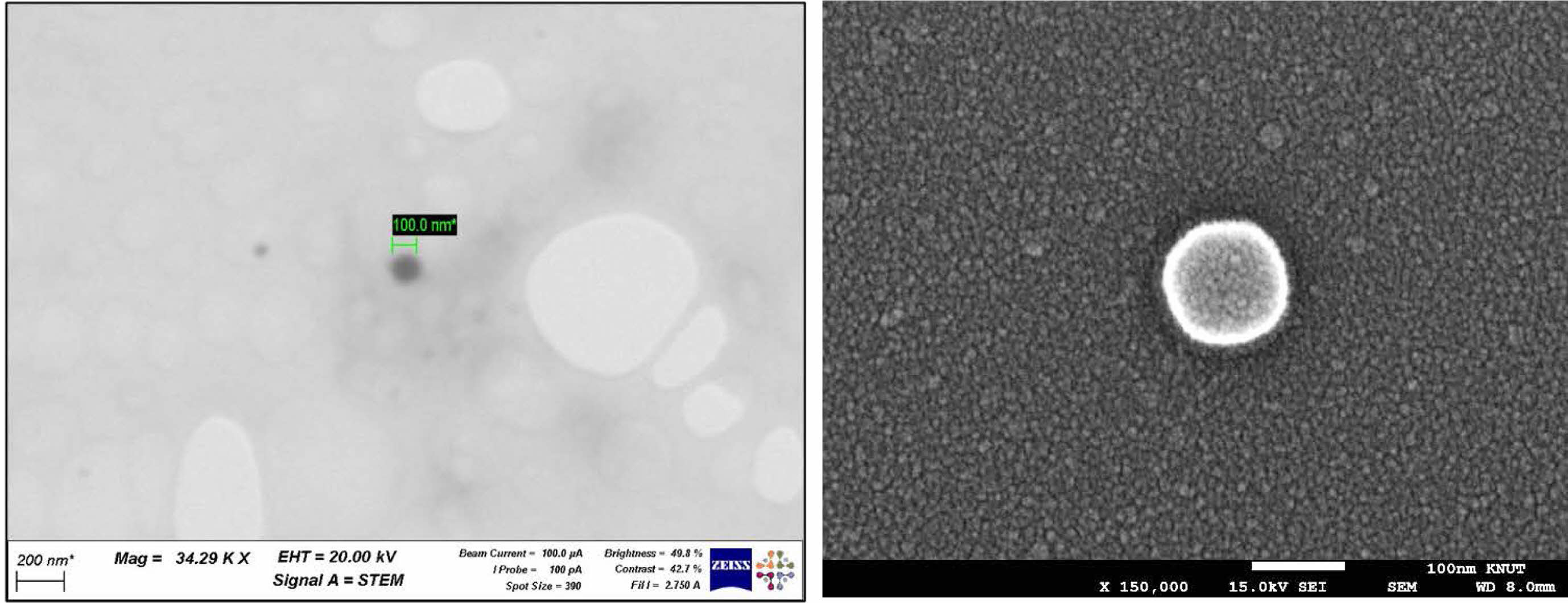
Morphology Study of TCA-cisplatin@liposomes. Dynamic light scattering was used to examine the prodrug nanoparticles' particle size distribution (Figure 2). A homogeneous population was suggested by the analysis's results, which showed a rather narrow distribution with an average diameter between 100 and 200 nanometers. Scanning electron microscopy (SEM) (Figure 3(b)) and scanning transmission electron microscopy (Figure 3(a)) were used for morphological characterisation, and the results showed that the cisplatin-conjugated nanoparticles were uniformly dispersed and spherical, measuring around 100 nanometers across. The creation of consistently nanoscale liposomes appropriate for pharmaceutical distribution has been made possible by the combination of dynamic light scattering and electron microscopy.
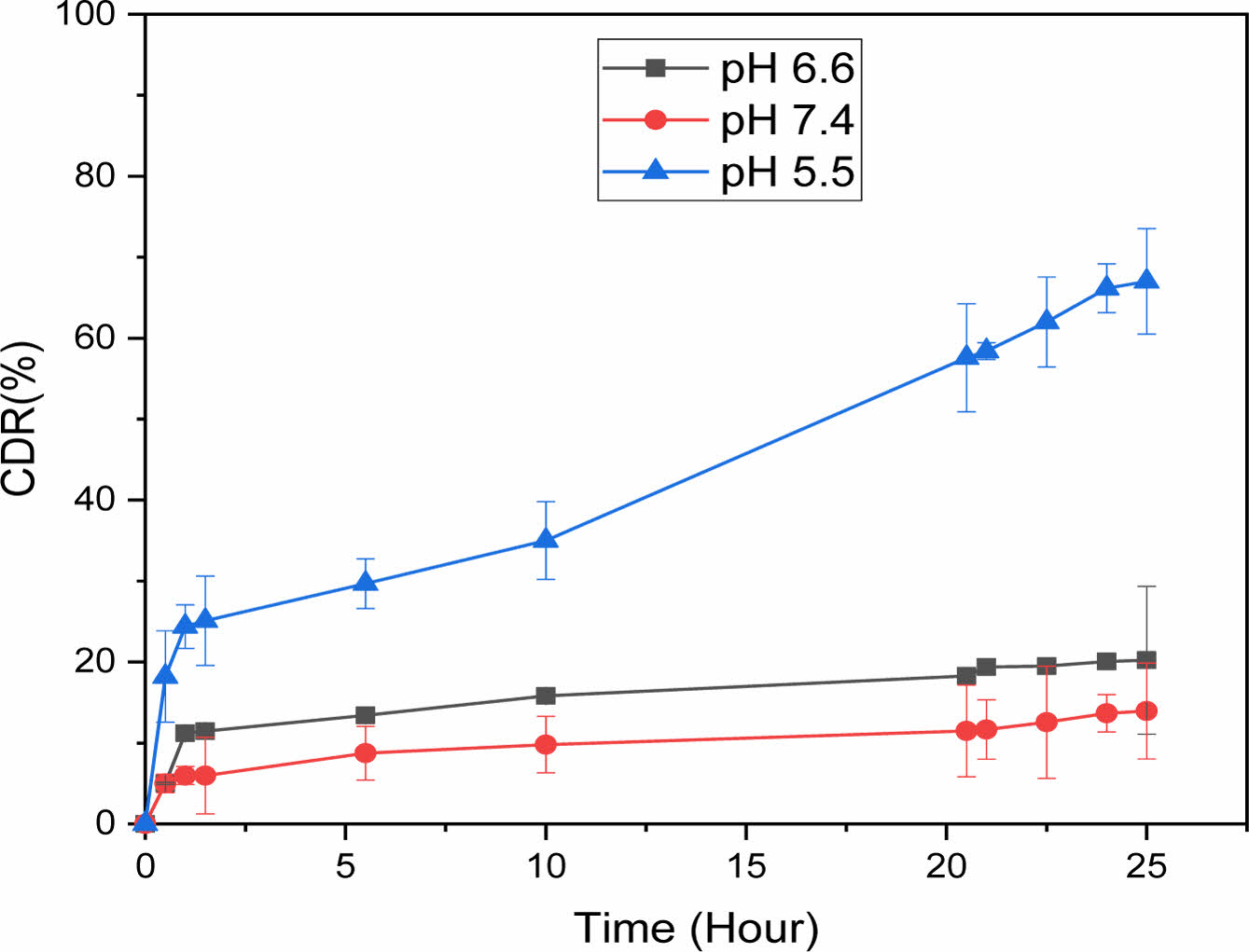
In Vitro Drug Release Study of TCA-cisplatin@liposomes. Controlled release represents considerable advantages in the drug delivery system compared to regular dosage forms.26 In this current study 67% of cisplatin was released from liposome in a controlled fashion during 24 hours at pH 5.5 which resembles the cancer cell environment. On the other hand, at pH 7.4 and pH 6.6 the drug release was minimal, 13.9% and 20% respectively. There was no burst release at any pH for TCA-cisplatin loaded liposomes (Figure 5).
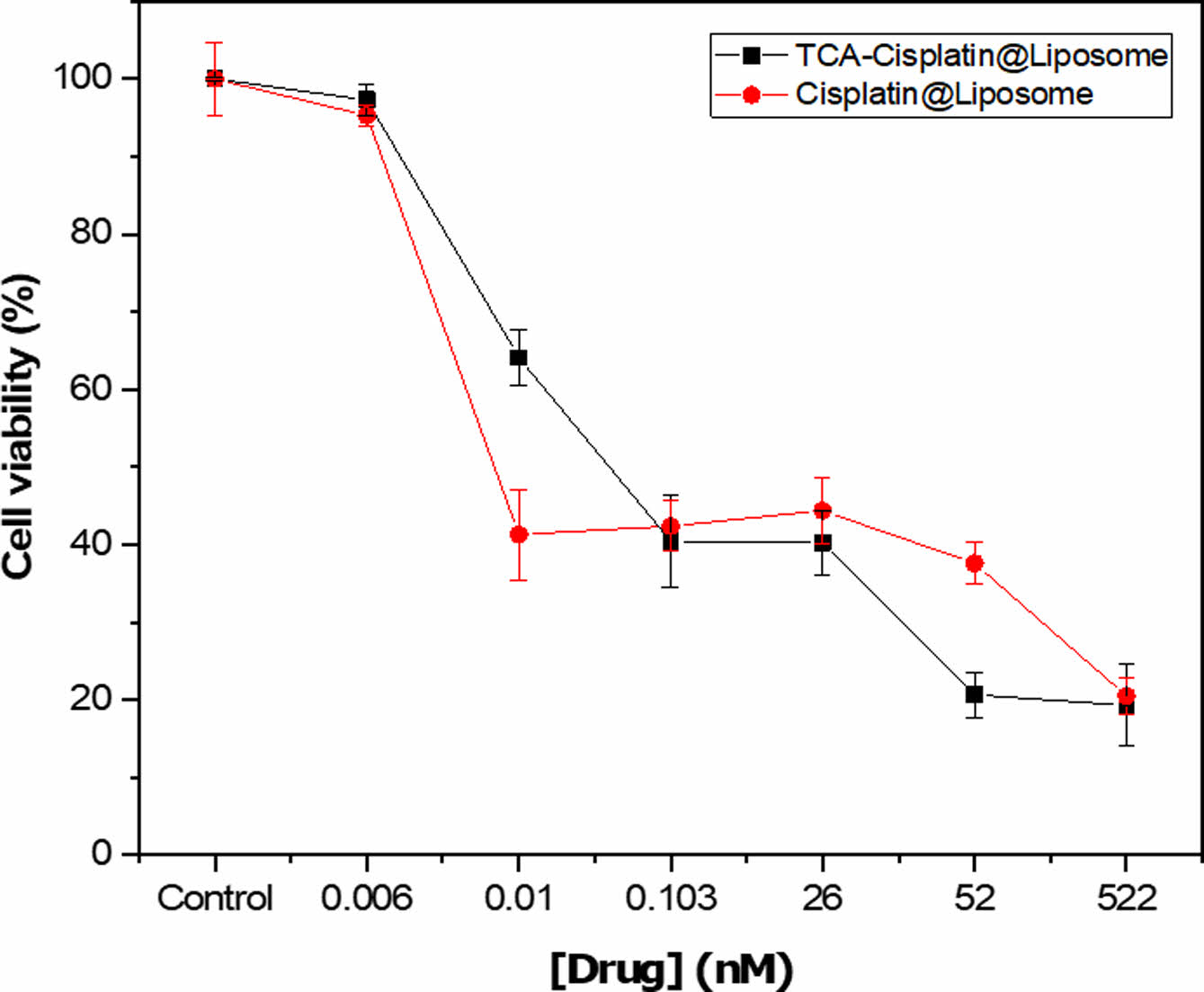
In Vitro Cytotoxicity of TCA-cisplatin@liposomes. In vitro cell cytotoxicity revealed that TCA-cisplatin loaded liposomes has toxicity on A549 cells nearly like cisplatin loaded liposomes during 48 hours. Below the concentration of 26 nM TCA-cisplatin represented 20% cell viability whereas cisplatin loaded liposome showed over 30%. The conjugate pro-drug liposome reveals almost similar in vitro cytotoxicity compared to cisplatin loaded liposomes. The IC50 was 0.05 and 0.004 of TCA-cisplatin liposome and cisplatin-liposome respectively (Figure 4).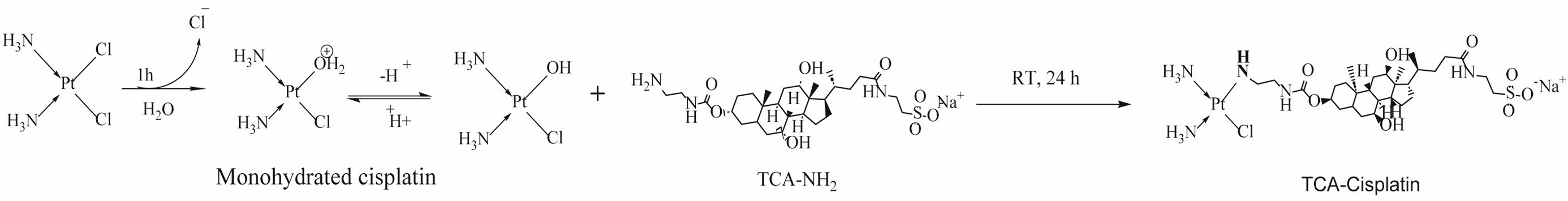 Scheme 1. Synthetic route for Cisplatin-TCA prodrug.
Scheme 1. Synthetic route for Cisplatin-TCA prodrug.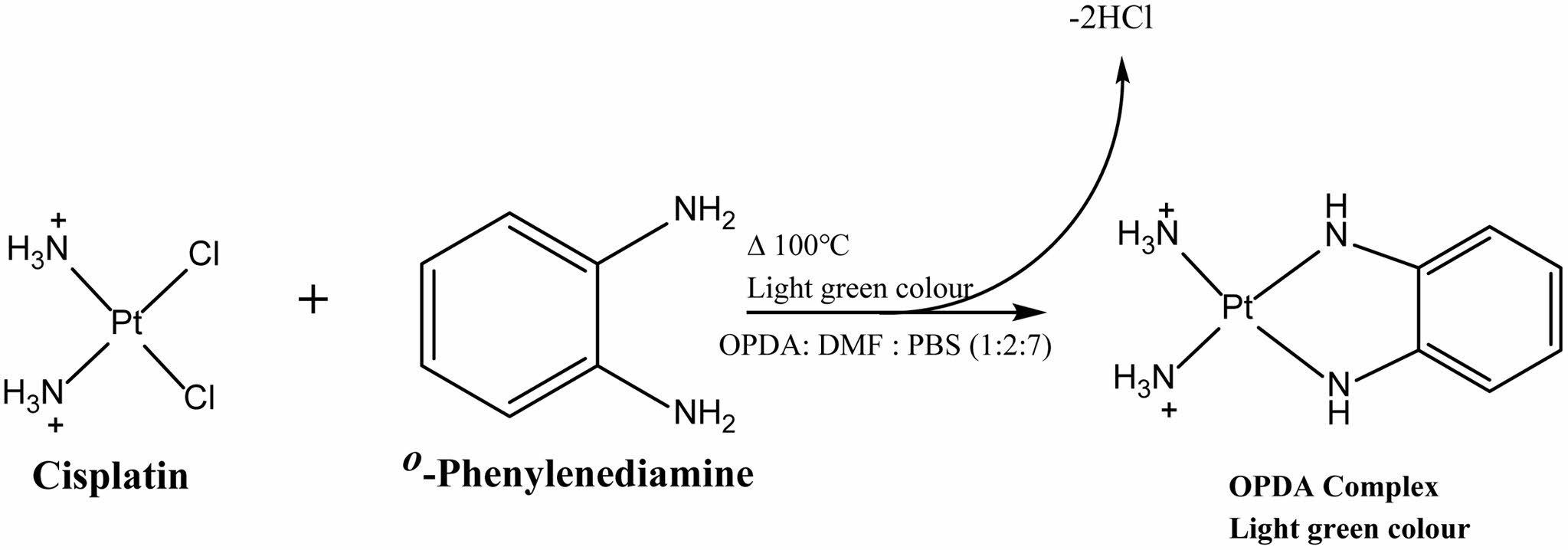
Scheme 2. Reaction between cisplatin and o-phenylenediamine.
|
Figure 1 1H NMR (400 MHz, DMSO-d6) analysis of (a) cisplatin; (b) TCA-NH2; (c) cisplatin-TCA prodrug. |
|
Figure 2 Particle size distribution measured by dynamic light scattering for TCA-cisplatin pro-drug. |
|
Figure 3 (a) Scanning transmission electron microscopy (STEM); (b) SEM images of L-CisTCA liposome. |
|
Figure 4 Cytotoxicity of cisplatin conjugated TCA-NH2 nano-particle loaded liposome. |
|
Figure 5 Cumulative release of cisplatin from liposomes at different pH at 37.5 ℃. |
Conjugating cisplatin with bile acids and encapsulating it in liposomes is an effective way to maximize its encapsulation efficiency, plus it maintains a high drug:lipid ratio. Liposomes encapsulating TCA-cisplatin were evaluated in vitro and showed increased entrapment efficiency and better drug loading. cisplatin was delivered by liposomes in a pH-dependent manner showing high drug release at pH 5.5, resulting in cancer cell-specific delivery of cisplatin. As well as showing cytotoxicity on lung cancer cells, the Complex TCA-cisplatin liposome behaved similarly to free drugs cisplatin liposomes. However, we are of the opinion that follow-up studies should include live/dead assays, an experiment that can complement the MTT assay. Even though the liposomal application was only in vitro, the release studies reveal that it is stable in mucous saliva at pH 6.6. It can therefore be developed into an oral drug with more development in the near future. Inevitably, it will have to pass through the gastrointestinal tract with low pH. Coating, modification techniques may be introduced to increase the stability of the carrier at low pH. However, it still needs further discussion whether the drug in TCA-cisplatin liposomes is likely to burst and release at the low pH of the stomach.
- 1. Rebecca, L. Siegel, Kimberly D Miller, Nikita Sandeep Wage, A Hmedin Jemal; Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17-48.
-

- 2. Woodman, C.; Vundu, G.; George, A.; Wilson, C. M. Applications and Strategies in Nanodiagnosis and Nanotherapy in Lung Cancer. Semin. Cancer Biol. 2021, 69, 349-364.
-

- 3. Kobir, M. E.; Ahmed, A.; Roni, M. A. H.; Chakma, U.; Amin, M. R.; Chandro, A.; Kumer, A. Anti-lung Cancer Drug Discovery Approaches by Polysaccharides: An in Silico Study, Quantum Calculation and Molecular Dynamics Study. J. Biomol. Struct. Dyn. 2023, 41, 6616-6632.
-

- 4. Imran Vhora, Nirav Khatri, Jagruti Desai, Hetal Paresh Thakkar; Caprylate-conjugated Cisplatin for the Development of Novel Liposomal Formulation. AAPS PharmSciTech. 2014, 15, 845-857.
-

- 5. Akimaru, K.; Auzenne, E.; Akimaru, Y.; Leroux, M. E.; Hayman, A. C.; Utsumi, T. Formulation and Antitumor Efficacy of Liposomal Caprylated-TNF-SAM2. Cytokines Mol. Ther. 1995, 3, 197-210.
- 6. Utsumi, T.; Hung, M. C.; Klostergaard, J. Preparation and Characterization of Liposomal-lipophilic Tumor Necrosis Factor. Cancer Res. 1991, 51, 3362-3366.
- 7. Cafaggi, S.; Russo, E.; Stefani, R.; Leardi, R.; Caviglioli, G.; Parodi, B. Preparation and Evaluation of Nanoparticles Made of Chitosan or N-trimethyl Chitosan and a Cisplatin-alginate Complex. J. Control. Release. 2007, 121, 110-123.
-

- 8. Yan, X.; Gemeinhart, R. A. Cisplatin Delivery From Poly(acrylic acid-co-methyl methacrylate) Microparticles. J. Control. Release. 2005, 106, 198-208.
-

- 9. Chandrababu Rejeeth, Tapas C. Nag, Soundarapandian Kannan; Cisplatin-functionalized Silica Nanoparticles for Cancer Chemotherapy. Cancer Nano 2013, 4, 127-136.
-

- 10. Hamman, J. H.; Demana, P. H.; Olivier, E. I. Targeting Receptors, Transporters and Site of Absorption to Improve Oral Drug Delivery. Drug. Target. Insights. 2007, 2, 71-81.
-

- 11. Khatun, Z.; Nurunnabi, M.; Reeck, G. R.; Cho, K. J.; Lee, Y.-K. Oral Delivery of Taurocholic Acid Linked Heparin–docetaxel Conjugates for Cancer Therapy. J. Controll. Release, 2013, 170, 74-82.
-

- 12. Kim, S. K.; Huh, J.; Kim, S. K.; Byun, Y.; Lee, D. Y.; Moon, H. T. Physicochemical Conjugation with Deoxycholic Acid and Dimethylsulfoxide for Heparin Oral Delivery. Bioconjug. Chem. 2011, 22, 1451-1458.
-

- 13. Khatun, Z.; Nurunnabi, M.; Reeck, G. R.; Cho, K. J.; Lee, Y.-K. Oral Delivery of Taurocholic Acid Linked Heparin-docetxel Conjugates for Cancer Therapy. J. Control. Release, 2013, 170, 74-82.
-

- 14. Andersson, A.; Hedenmalm, H.; Elfsson, B.; Ehrsson, H. Determination of the Acid Dissociation Constant for Cis-diammineaquachloroplatinum (II)ion. A Hydrolysis Product of Cisplatin. J. Pharm. Sci. 1994, 83, 859-862.
-

- 15. Ramos, Y.; Hernandez, C.; Fernandez, L. A.; Bataller, M.; Veliz, E.; Small, R. Optimization of a HPLC Procedure for Simultaneous Determination of Cisplatin and the Complex Cis, Cis, Trans-diamminedichlorodihydroxoplatinum (IV) in Aqueous Solutions. Quim. Nova, 2011, 34, 1450-1454.
-

- 16. Anilanmert, B.; Yalcin, G.; Arioz, F.; Dolen, E. The Spectrophotometric Determination of Cisplatin in Urine, Using o-Phenylenediamine as Derivatizing Agent. Anal Lett. 2001, 34, 113-123.
-

- 17. Munawara, I.; Shi, Y.; Koneru, B.; Patel, A.; Dang, M. H.; Di Pasqua, A. J.; Balkus Jr, K. J. Nitric Oxide-and Cisplatin-releasing Silica Nanoparticles for Use Against Non-small Cell Lung Cancer. J. Inorg. Biochem. 2015, 153, 23-31.
-

- 18. Werner, M. E.; Copp, J. A.; Karve, S.; Cummings, N. D.; Sukumar, R.; Li, C. Folate-targeted polymeric Nanoparticle Formulation of Docetaxel is An Effective Molecularly Targeted Radiosensitizer with Efficacy Dependent on the Timing of Radiotherapy. ACS Nano 2011, 5, 8990-8998.
-

- 19. Yan, X.; Gemeinhart, R. A. Cisplatin Delivery From Poly(acrylic acid-co-methyl methacrylate) Microparticles. J. Control. Release. 2005, 106, 198-208.
-

- 20. Ebrahimi Shahmabadi, H.; Akbarzadeh, A.; Mokhtari, M. J.; Mortazavi, M.; Ghasemi, S.; Mohammadi, H.; Dou, S. K. B. In vitro Evaluation of the Effects of Acetone, on the Potency of Cisplatin: is it a Good Candidate for Cisplatin Carrier Preparation. Biotechnol. Pharm Res. 2012, 3, 137-140.
- 21. Ebrahimi Shahmabadi, H.; Movahedi, F.; Koohi Moftakhari Esfahani, M.; Alavi, S. E.; Eslamifar, A.; Mohammadi Anaraki, G.; Akbarzadeh, A. Efficacy of Cisplatin Loaded Polybutyl Cyanoacrylatenanoparticles on the Glioblastoma. Tumor Biol. 2014, 35, 4799-4806.
-

- 22. Tomas Zimmermann, Jerzy Leszczynski, Jaroslav V Burda. Activation of the Cisplatin and Transplatin Complexes in Solution with Constant pH and Concentration of Chloride Anions; Quantum Chemical Study. J. Molecul. Modeling 2011, 17, 2385-2393.
-

- 23. Kastantin, M.; Ananthanarayanan, B.; Karmali, P.; Ruoslahti, E.; Tirrell, M. Effect of the Lipid Chain Melting Transition on the Stability of DSPE-PEG(2000) Micelles. Langmuir 2009, 25, 7279-7286.
-

- 24. Zhong, J.; Huang, H.-L.; Li, J.; Qian, F.-C.; Li, L.-Q.; Niu, P.-P.; Dai, L.-C. Development of Hybrid-type Modified Chitosan Derivative Nanoparticles for the Intracellular Delivery of Midkine-siRNA in Hepatocellular Carcinoma Cells. Hepatobil. Pancreat. Dis. Int. 2015, 14, 82-89.
-

- 25. Liu, M.; Zhang, X.; Yang, B.; Deng, F.; Ji, J.; Yang, Y.; Huang, Z.; Zhang, X.; Wei, Y. Luminescence Tunable Fluorescent Organic Nanoparticles from Polyethyleneimine and Maltose: Facile Preparation and Bioimaging Applications. RSC Adv. 2014, 4, 22294-22298.
-

- 26. Sun, Y.; Peng, Y.; Chen, Y.; Shukla, A. J. Application of Artificial Neural Networks in the Design of Controlled Release Drug Delivery Systems. Adv. Drug Deliv. Rev. 2003, 55, 1201-1215.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2024 Impact Factor : 0.6
- Indexed in SCIE
 This Article
This Article
-
2025; 49(5): 570-578
Published online Sep 25, 2025
- 10.7317/pk.2025.49.5.570
- Received on Jan 30, 2025
- Revised on Apr 21, 2025
- Accepted on Apr 21, 2025
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
- Conflict of Interest
- Supporting Information
Introduction
Experimental
Results and Discussion
Conclusion
- References
Shared
 Correspondence to
Correspondence to
- Yong-kyu Lee
-
**Department of Chemical and Biological Engineering, Graduate School, Korea National University of Transportation, Chungju 27469, Korea
***4D Convergence Technology Institute, Korea National University of Transportation, Chungju 7469, Korea - E-mail: leeyk@ut.ac.kr
- ORCID:
0000-0001-8336-3592









 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.