- Catalyst Effect on the Self-Healing Properties of Bio-Based Diels-Alder Polyurethanes
Heru Santoso*, **,†
 , Sabrina Aufar Salma**, Frita Yuliati**, Safira Dwi Cahyani***, and Sumarno Sumarno*,†
, Sabrina Aufar Salma**, Frita Yuliati**, Safira Dwi Cahyani***, and Sumarno Sumarno*,† 
*Department of Chemical Engineering, Institut Teknologi Sepuluh Nopember (ITS), Surabaya 60111, Indonesia
**Research Center for Polymer Technology, National Research and Innovation Agency (BRIN), KST BJ. Habibie 460 building, South Tangerang 15314, Indonesia
***Chemistry Department, Syarif Hidayatullah State Islamic University (UIN), Jakarta 15412, Indonesia- 바이오 기반 Diels-Alder 폴리우레탄의 자가치유 특성에 대한 촉매 효과 연구
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
This study investigates the impact of dibutyltin dilaurate (DBTDL) and dibutyltin diacetate (DBTDA) catalysts on the properties of bio-isocyanate trimer-based polyurethanes, focusing on their Diels-Alder (DA) self-healing mechanism. Polyurethane prepolymer, derived from polyethylene glycol and pentamethylene diisocyanate trimer, was functionalized with furfuryl amine and crosslinked with bismaleimide to form self-healing networks. Fourier-transform infrared spectroscopy (FTIR) analysis confirmed successful bond formation in both catalyst systems, with no significant chemical structural differences. However, differential scanning calorimetry (DSC) analysis showed DBTDL resulted in superior DA crosslinking, as indicated by a greater reduction in the PEG melting enthalpy and increased gel content compared to DBTDA. The thermal reversibility of the DA bonds was also demonstrated. Both qualitative (optical microscopy) and quantitative (tensile testing) assessments revealed over 100% self-healing efficiency for both catalysts, with DBTDL-catalyzed polyurethanes exhibiting better tensile strength recovery. These findings suggest DBTDL is more effective in enhancing DA crosslinking and polymer network recovery, thereby significantly improving the self-healing performance of bio-based polyurethanes. This emphasizes the importance of optimal catalyst selection for maximizing the mechanical properties and healing capabilities in sustainable coating applications.
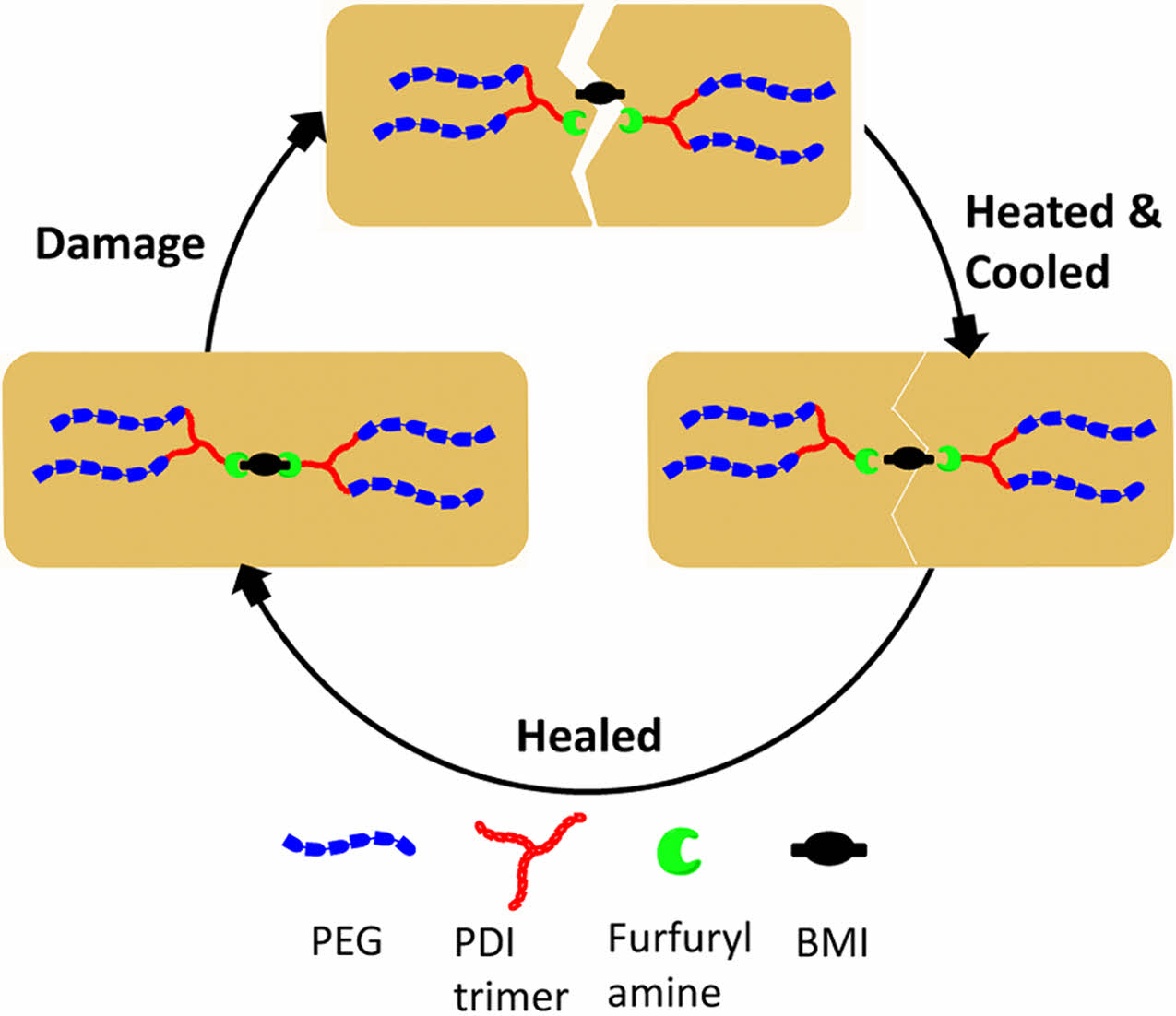
Self-healing polyurethane materials successfully synthesized via the Diels–Alder reaction using dibutyltin dilaurate (DBTDL) and dibutyltin diacetate (DBTDA) as catalysts. The self-healing performance was systematically assessed through microscopic analysis to observe surface recovery and tensile testing to evaluate the restoration of mechanical strength. Differential scanning calorimetry (DSC) analysis revealed that thermal treatment at 70 ℃for 24 hours triggered the retro-Diels–Alder reaction, as evidenced by the reappearance of an endothermic peak corresponding to the reformation of furan–maleimide covalent bonds.
Keywords: self-healing polyurethane, Diels-Alder reaction, biobased, isocyanate trimer, catalyst, dynamic crosslink.
This research was financially supported by the National Research and Innovation Agency of Indonesia (BRIN) through its Nanotechnology and Materials Program House (Grant No. 3/III.10/HK/2023).
The authors have no conflicts of interest relation to this research.
More detailed information about swelling degree, and gel content tests is provided in the Supporting Information file. The materials are available via the Internet at http://journal.polymer-korea.or.kr.
PK_2025_049_06_818_Supporting_Information.pdf (360 kb)
Supplementary Information
Polyurethane (PU) is a highly adaptable polymer commonly used in a wide range of industries, such as adhesives, coatings, automotive, construction, and healthcare.1-3
Its main advantage lies in its formulation flexibility, which enables the tailoring of mechanical, thermal, and chemical properties through adjustments in polyols and isocyanates.4 However, PU is prone to mechanical and environmental degradation, resulting in performance loss and reduced material lifespan due to hard-to-detect microcracks.5 Traditional repair methods are ineffective in addressing these micro-cracks, highlighting the potential of developing PU with self-healing capabilities as a viable solution and an expanding research area.
Self-healing mechanisms are inspired by regenerative processes found in living organisms. Over the past two decades, several self-healing polymers have been developed, with most still relying on petrochemical-based materials.6 In response to the limitations of fossil resources and their environmental consequences, research has increasingly shifted toward bio-based materials—those derived from living organisms that offer more sustainable environmental options.7 The development of bio-based PU with dynamic covalent structures contributes to a circular economy by facilitating recycling and extending the material’s lifespan from the design phase.8 Bio-based diisocyanates have become a key alternative in polymer research to promote sustainability. One such promising option is the pentamethylene diisocyanate trimer (PDI-t), a straight-chain aliphatic diisocyanate derived from renewable sources. PDI is low in toxicity and has been commercialized by Covestro for applications in coatings, foams, and adhesives. It is produced from corn starch, animal feed, and non-food industrial crops, ensuring no direct competition with food production.9–11
Self-healing materials can regain their original properties either independently or through the application of external stimuli. A particularly effective method for achieving this restoration is the Diels–Alder (DA) reaction, which facilitates the reversible formation and cleavage of covalent bonds in response to temperature changes, thereby enabling damage repair through repeated heating. This attribute renders the DA reaction exceptionally suitable for polymer applications because, it mitigates the need for extreme thermal conditions. Numerous studies have concentrated on the development of self-healing PUs utilizing the DA reaction, primarily employing two methodologies: the incorporation of furan groups into PU prepolymer that subsequently react with bismaleimide, and the synthesis of PU prepolymer that contain maleimide groups, which are later engaged in reactions with bifunctional furan compounds.5
PU synthesis is achieved through the reaction of isocyanates, either diisocyanates or polyisocyanates, with polyols to form urethane bonds. Trimer isocyanates, such as isocyanurate, biuret, and allophanate, each containing three isocyanate groups per molecule, serve as crosslinking agents. This results in the formation of a complex polymer network that enhances the thermal stability, chemical resistance, and mechanical properties. The unique ring structure of isocyanurate notably increases the thermal stability and chemical resistance of PU.12 Aliphatic trimer isocyanates are particularly favored for coatings because of their low toxicity, excellent thermal stability, superior mechanical strength, and high resistance to weathering.13
The choice between aromatic and aliphatic isocyanates in the PU synthesis is critical and should be tailored to the intended application.14 Researchers have widely employed catalysts and isocyanate trimers in the synthesis of DA self-healing PUs, with dibutyltin dilaurate (DBTDL) catalysts commonly used alongside isocyanates like hexamethylene diisocyanate trimer (HDI-t)15–20
and isophorone diisocyanate trimer (IPDI-t).21 While the use of dibutyltin diacetate (DBTDA) catalysts is less prevalent, it has been applied in research with isophorone diisocyanate trimer (IPDI-t).22,23
The literature reveals that, although there has been significant research into self-healing PUs based on DA reactions using isocyanate trimers such as HDI-t, and IPDI-t and some preliminary studies on PDI-t, there is still a gap in integrating PDI-t into the self-healing mechanism. The lack of comprehensive, systematic studies on the synergistic effects of PDI-t as isocyanate components in self-healing PUs highlights an important area for further investigation.
This research investigates the development of a self-healing PU using a bio-based PDI-t as an isocyanate via the DA reaction. The initial step involves synthesizing the PU prepolymer from the PEG and PDI-t, adjusting for reaction time and temperature variations. Subsequently, this prepolymer reacted with the furfuryl amine to produce the furan-terminated PU. The formation of the self-healing PU is formed through the cross-linking of PU-FA with bismaleimide, again employing the DA reaction. Furthermore, this study explores the effects of DBTDL and DBTDA catalysts on the mechanical properties and healing efficiency of the resultant PU. The findings are intended to advance the development of eco-friendly PUs, specifically targeting their applications in self-healing coatings.
Materials. Polyethylene glycol - PEG (MW 4000 g/mol), DBTDL (USA), DBTDA (USA), furfurylamine 99% (USA), and bismaleimide (BMI from Germany) were purchased from Sigma-Aldrich and used as received. The bio-based pentamethylene diisocyanate trimer (PDI-t), commercially designated as Desmodur® eco N7300, was provided by Covestro Indonesia. Dimethylformamide (Germany), used as the reaction solvent, was sourced from Merck.
Synthesis of a Self-healing PU. PEG (6.4 g, 1.6 mmol), PDI-t (2.2 g, 4.8 mmol), and DBTDL catalyst were dissolved in 10 mL of DMF and stirred in a 250 mL three-neck flask equipped with a mechanical stirrer and reflux condenser. For comparative consistency, the same procedure was conducted using DBTDA as the catalyst. The reaction mixture was heated to 80 ℃ and maintained for 1 hour to synthesize a PU prepolymer with an NCO/OH ratio of 3. Then, it was cooled in an ice-water bath. Furfuryl amine (0.68 g, 7 mmol) was pre-dissolved in 2 mL of DMF and added dropwise to the prepolymer solution over 10 minutes. After stirring for 30 minutes, the mixture was reheated to 65 ℃ and held for another hour. Upon cooling to room temperature, the furfuryl amine-functionalized prepolymer (PU-FA) was slowly mixed with bismaleimide (BMI, 1.8 g, 5 mmol) and heated at 65 ℃ for an additional hour. The final product was then cast onto a PTFE sheet and dried in a vacuum oven at 60 ℃ for 24 hours. Table 1 provides the details of the catalyst treatments and formulations.
Characterization. A series of characterizations was performed to evaluate the chemical structure and physical properties of the self-healing PU materials. Fourier-transform infrared spectroscopy (FTIR) was employed to monitor changes in the functional groups throughout the DA reaction. Spectra were obtained using a Bruker Tensor 27 spectrometer equipped with an ATR accessory, collecting 64 scans over a wavenumber range of 4000–500 cm-1. 1H NMR measurements were carried out on an Oxford Instruments X-Pulse Benchtop NMR Spectrometer (60 MHz), utilizing DMSO-d6 as the deuterated solvent. Thermal transitions, including the melting temperature and fusion enthalpy, were analyzed by differential scanning calorimetry (DSC) using a PerkinElmer DSC 8000. Measurements were conducted within a temperature range of 0 ℃ -190 ℃ to assess the thermal behavior of the samples.
The density of the cross-linked PU samples was determined by pycnometry using distilled water as the displacement medium. Detailed procedures for measuring the swelling degree and gel content measurements are provided in the Supporting Information.
The self-healing properties of PU-DA were assessed through both qualitative and quantitative methods. A digital microscope was used to examine knife-cut cracks. Samples with 0.5 mm scratches underwent a heating process at 130°C for 10 minutes and then at 130 ℃ for 2 h, followed by a 24 h incubation period at 70 ℃. The tensile strength recovery was evaluated using a Shimadzu AG-X Plus 50 kN universal testing machine. The self-healing efficiency was determined based on the tensile strength recovery in accordance with the ASTM D638 type 4 standards.
|
Table 1 Formulation Parameters for the DA PU Synthesis |
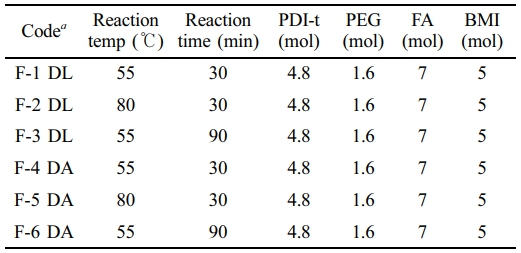
a The DL code indicates the use of the DBTDL catalyst, whereas the DA code represents the use of the DBTDA catalyst. |
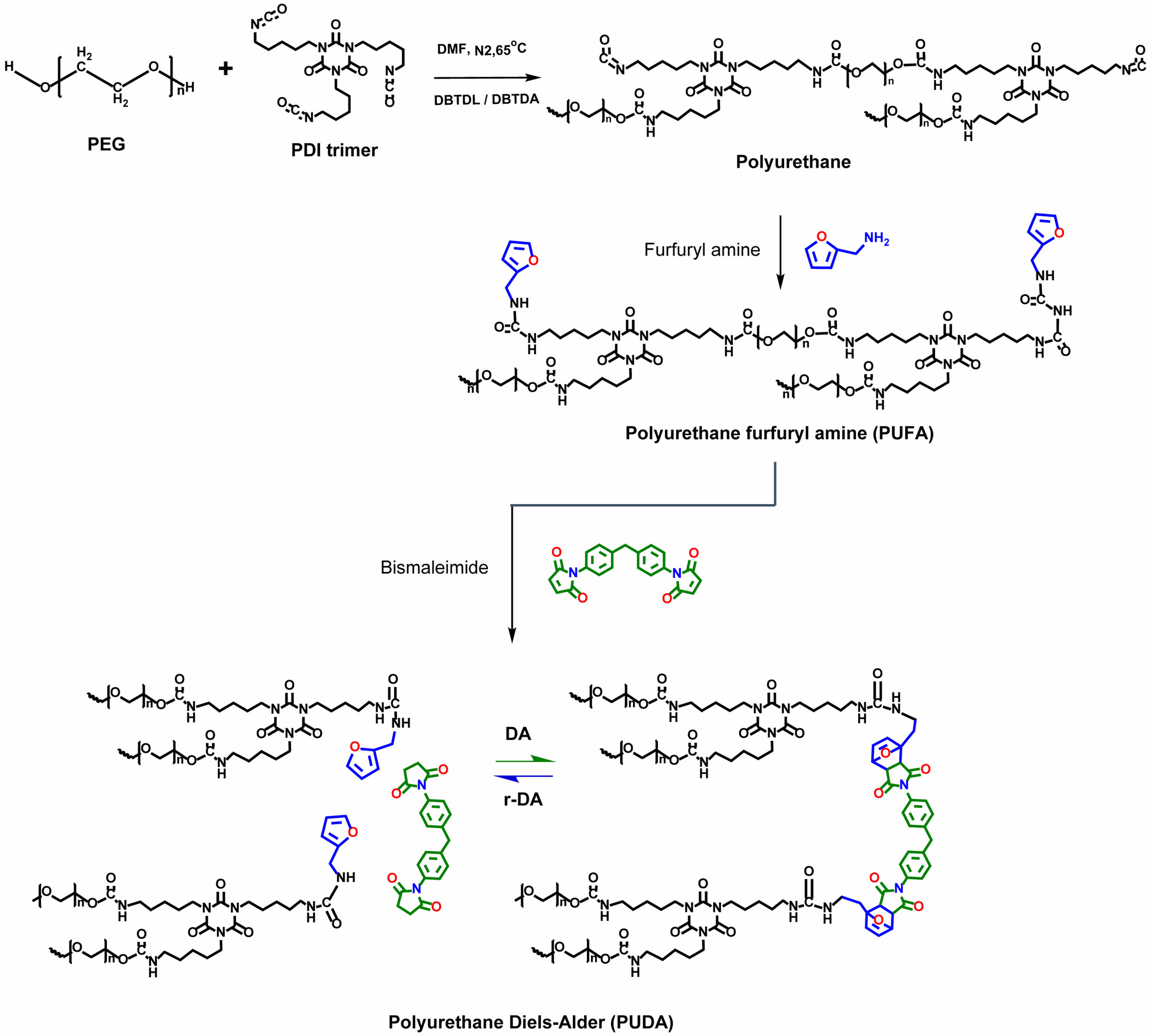
A self-healing PU was synthesized through a systematic series of steps. The initial phase involved the creation of an isocyanate (NCO)-terminated prepolymer, which was achieved by reacting PEG with an excess of PDI-t, with DBTDL serving as the catalyst; DBTDA was also evaluated as an alternative catalyst. Subsequently, the prepolymer was reacted with furfuryl amine to yield a furan-terminated PU. The final stage involved the crosslinking of the furan-terminated PU with BMI via a DA reaction, thereby yielding the self-healing PU network identified as PU-DA. A detailed illustration of the synthesis mechanism is provided in Figure 1.
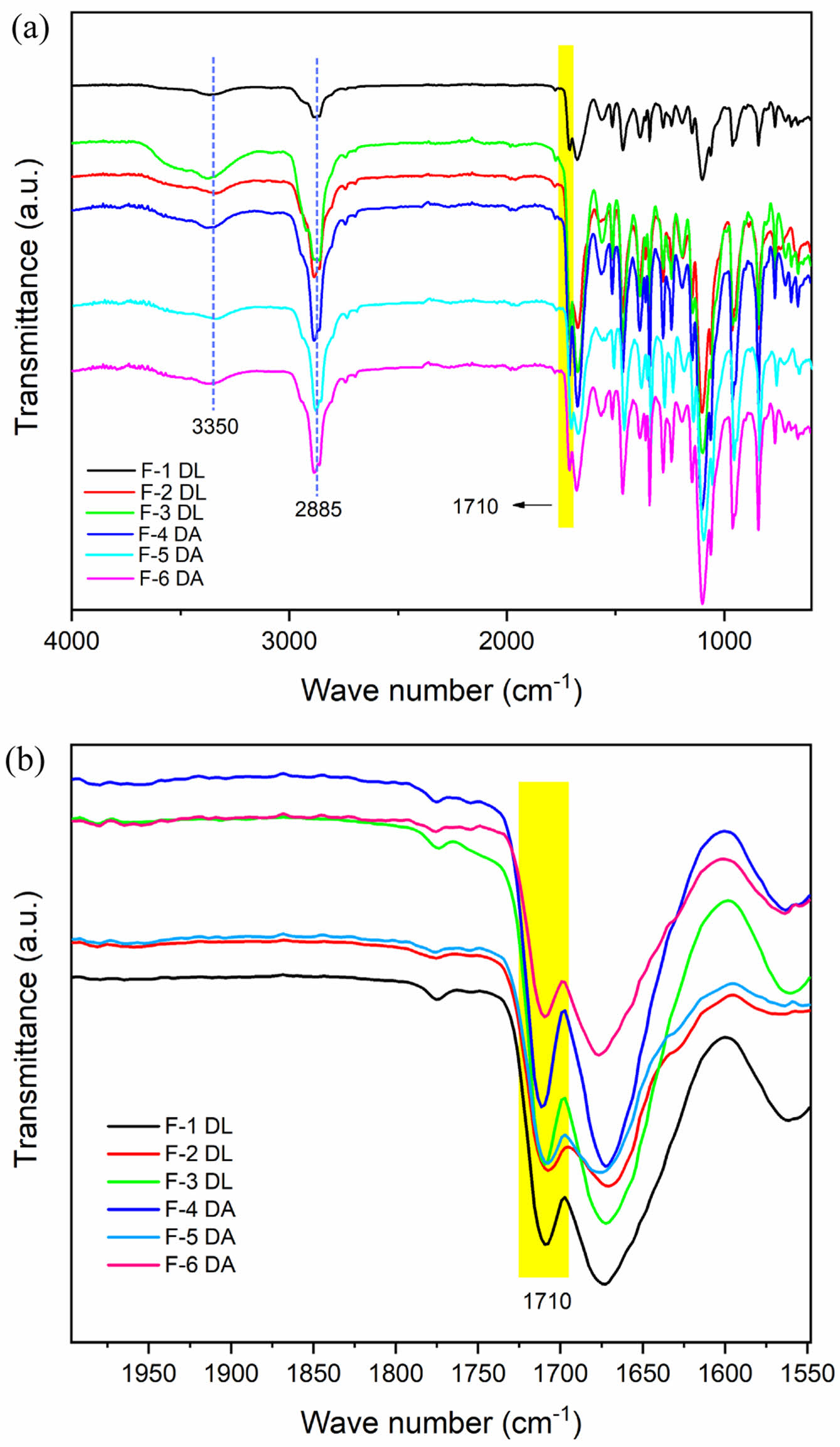
FTIR analysis was conducted to observe the changes in the functional groups within the self-healing PU materials caused by the DBTDL and DBTDA catalysts, with a particular emphasis on the formation of furan and maleimide groups during the DA reaction. Figure 2 shows that the FTIR spectra for both catalysts exhibited similar patterns. The absence of the 2280 cm-1 peak, which is typically associated with -N=C=O stretching, indicates that the isocyanate group from the prepolymer had completely reacted with the hydroxyl groups of PEG, leading to the formation of urethane groups.24 The peak at 2858 cm-1 was attributed to the CH2 group, while the band at 3334 cm-1 corresponded to the stretching of the hydrogen-bonded urethane N−H.25 The appearance of a new absorption band at 1710 cm-1 (C=O cycloaddition) confirmed the formation of furan-maleimide cross-links, which are characteristic of the DA reaction.26,27
The appearance of additional peaks at 1010, 810, and 736 cm-1 in the FTIR spectrum (Figure S1(a)) provides evidence for the presence of furan ring structures in PU–furan (PU-FA).28,29
Following the reaction with BMI, a noticeable decrease in the intensity of these peaks (Figure S1(b)) suggests the involvement of the furan ring in the DA reaction, thereby indicating successful cross-linking between PU–furan and BMI.30
In addition, there was no considerable difference between the DBTDL and DBTDA catalysts in their effectiveness at activating the furan and maleimide groups for cross-linking during the production of thermo-reversible PU.
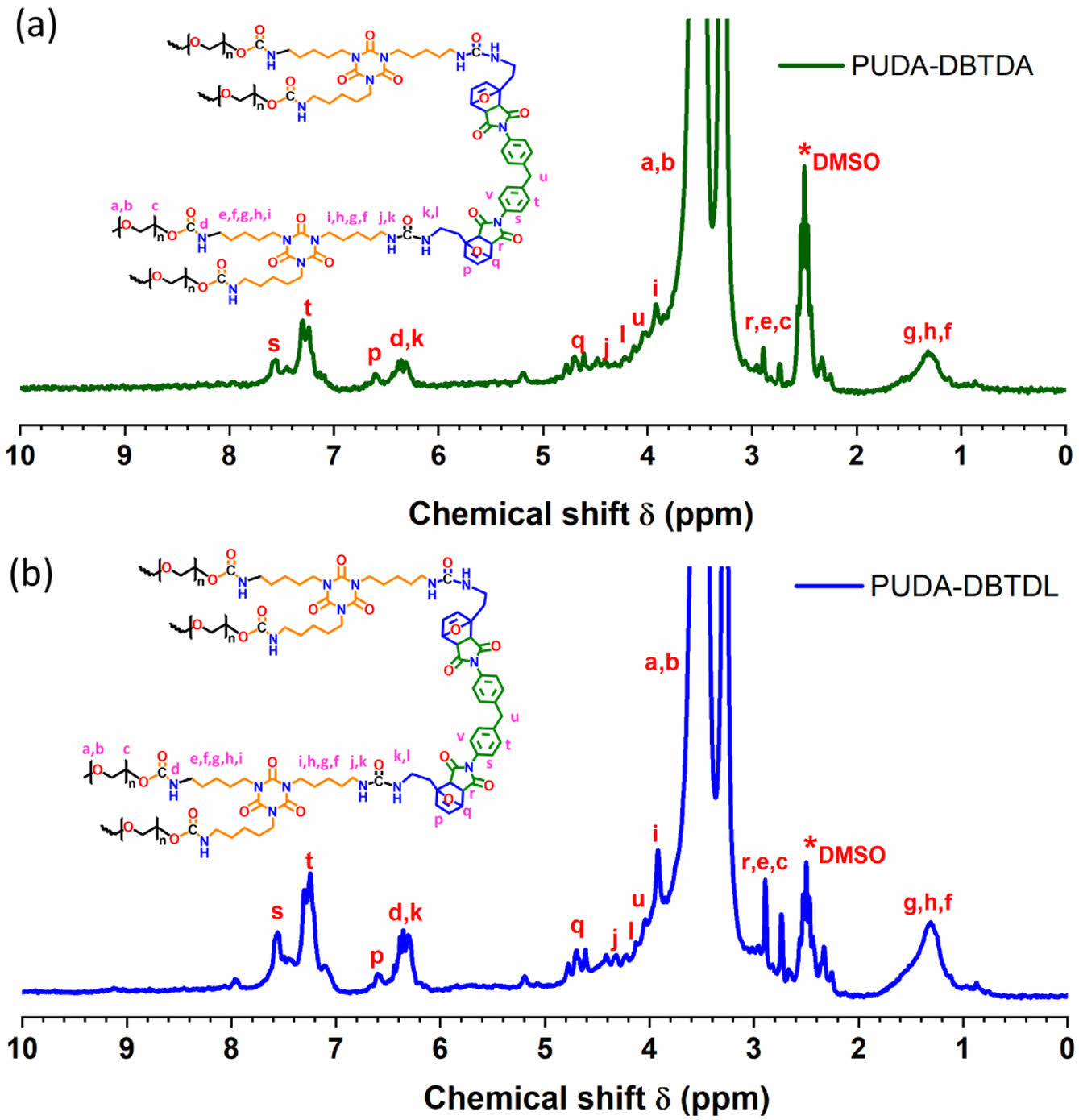
As shown in Figure 3, the 1H NMR spectra confirm the presence of maleimide functional groups, indicating successful crosslinking in the PU-DA structure. The formation of the DA adduct is evidenced by characteristic peaks at 2.95–2.96 ppm (r), 5.18–5.19 ppm (q), and 6.60–6.61 ppm (p), with the methylene protons of maleimide observed at 4.00 ppm (u). Despite using different catalysts, both PU samples exhibit nearly identical chemical shift patterns, suggesting comparable chemical structures. The 1H NMR analysis was performed after completion of the DA reaction.
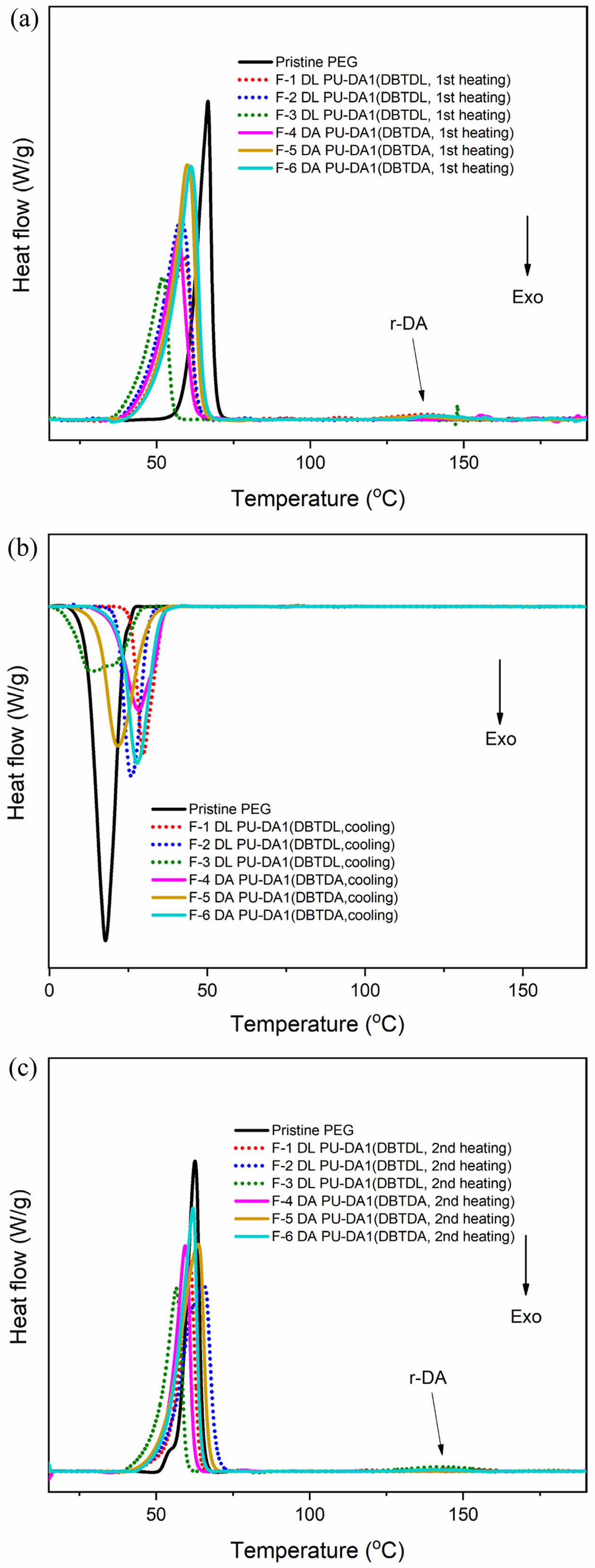
The thermal properties of the PU-DA synthesized with both catalysts were assessed using DSC. The findings indicated that the melting temperature of the self-healing PU was reduced compared to the original the PEG, which had a melting point of 65.14 ℃. This suggests that the establishment of DA cross-links disrupts the structure of PEG crystals, leading to a reduction in the number of crystal segments and a subsequent decrease in the melting temperature of the original PEG crystals.31
The DSC data for the first (ΔH1) and second (ΔH2) heating melting enthalpies of the self-healing PU formulations with both catalysts showed a decrease compared to pristine PEG, confirming that the PEG segment crystals were disrupted due to the formation of DA cross-links.32
For the DA PU formulation with the DBTDL catalyst, the decrease in the melting enthalpy (ΔH) was more significant than that observed with the DBTDA catalyst (see Table S1), indicating that the formation of DA cross-links with the DBTDL catalyst is more pronounced than with the DBTDA catalyst. In addition, the cooling DSC curve (Figure 4(b)) shows that the exothermic peak of pristine PEG occurs at a crystallization temperature lower than that of the self-healing PU, reflecting the reduced PEG crystal content. The DSC curves for both the first and second heating cycles (Figures 4(a) and 4(c)) display endothermic peaks near 140 ℃, which corresponds to the dissociation of the furan and maleimide bonds, a characteristic feature of the retro-DA reaction.33
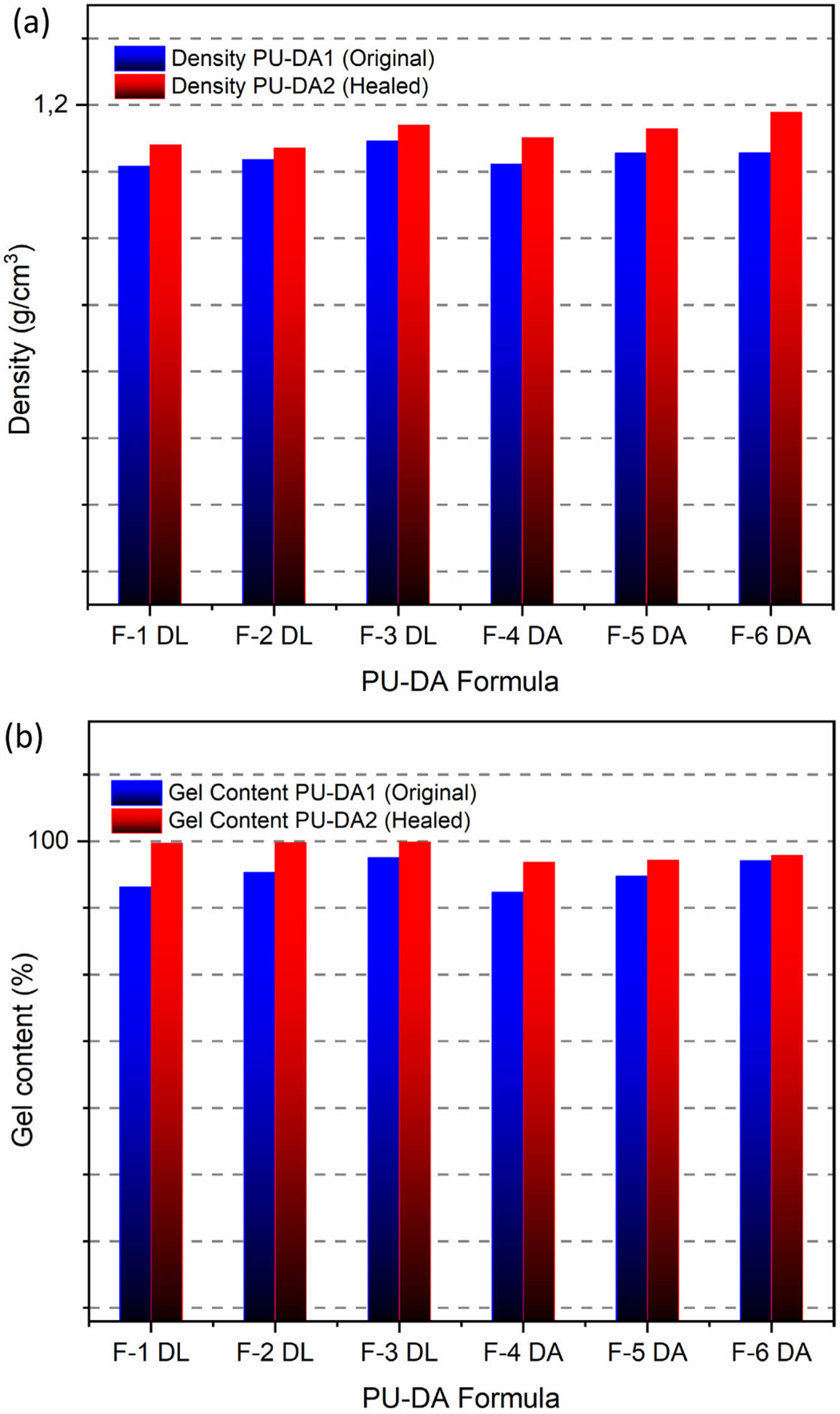
Figure 5 shows that the self-healing PU with both catalysts displayed increased density and gel content after healing (PU-DA2), implying the formation of more DA cross-links. Furthermore, the DBTDL catalyst resulted in a higher gel content than DBTDA, indicating a greater degree of DA cross-linking within that system. This observation is supported by the DSC measurements of the PEG melting enthalpy (see Table S1), which showed a lower value with the DBTDL catalyst, indicating a higher density of the resulting DA cross-links.
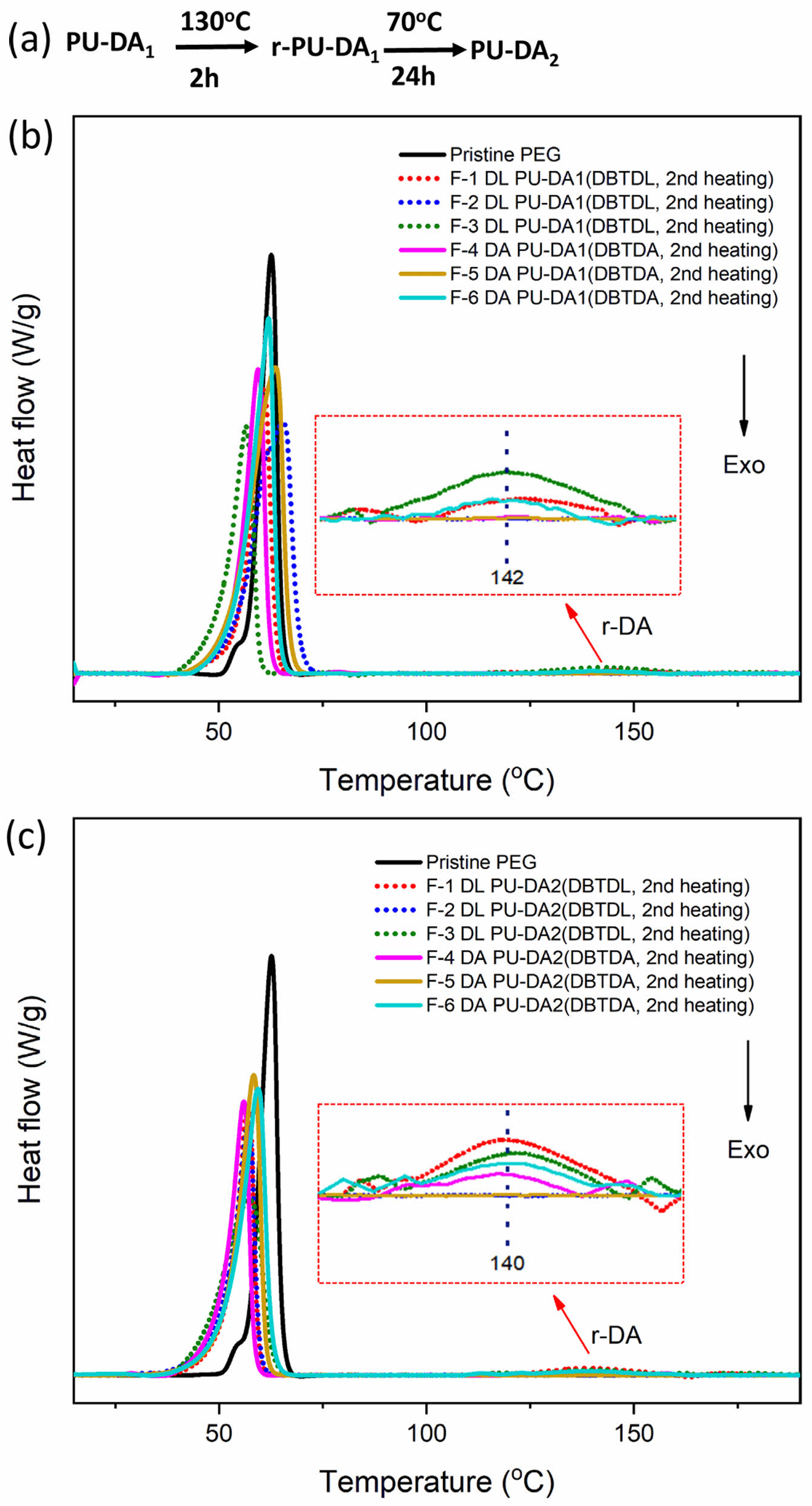
The DSC technique provides key insights into the material characteristics that can be altered through thermal processes. DSC measurements were performed during the second heating cycle of both untreated and 130 ℃-treated samples (for 2 h) to assess the thermal reversibility of DA PU synthesized with both catalysts. This approach aimed to explore the possibility of retro-DA reactions, followed by further heating at 70 ℃ for 24 h to enhance DA cross-linking (Figure 6(a)). The resulting DSC curves are presented in Figures 6(b) and 6(c), respectively.
Figure 6(b) shows the DSC curves of the PU-DA formulations with both catalysts before thermal treatment (original), showing an endothermic peak at 142 ℃, which is associated with the melting of the PEG crystal segments in the PU matrix formed through the DA reaction. This peak indicates damage to the DA bond caused by heat treatment in the original self-healing PU synthesis formula (PU-DA1), observed in samples F-1 DL PUDA1 and F-3 DL PUDA1 for the DBTDL catalyst, as well as in sample codes F-4 DA PUDA1 and F-6 DA PUDA1 for the DBTDA catalyst. After heat treatment at 130 ℃ and cooling at 70 ℃ for 24 h (PU-DA2), DSC analysis (Figure 4(c)) revealed the reappearance of the endothermic peak at 140 ℃, indicating the retro-DA reaction, where the DA bond decomposes into furan and maleimide upon heat absorption. Both catalysts exhibit the same behavior as in the PU-DA1 sample, with retro-DA bond formation occurring in the DBTDL catalyst (sample codes F-1 DL PUDA2 and F-3 DL PUDA2) and in the DBTDA catalyst (sample codes F-4 DA PUDA2 and F-6 DA PUDA2). These results demonstrate that the DA reaction between furan PU and BMI is reversible when processed at 70 ℃, facilitating the reformation of DA bonds.
The use of the DBTDL catalyst in the synthesis of DA PU results in a higher density of DA cross-links than the DBTDA catalyst. This is supported by the observed decrease in PEG melting enthalpy in PU-DA2 versus PU-DA1, as measured by DSC (see Figure S2). The reduction in melting enthalpy is attributed to the formation of cross-links in PU-DA2, which restricts the movement of PEG crystal segments.34
The decrease in enthalpy signifies the reformation of DA bonds during the 24 h heating at 70 ℃, confirming the consistent thermal reversibility of the synthesized PU-DA samples.
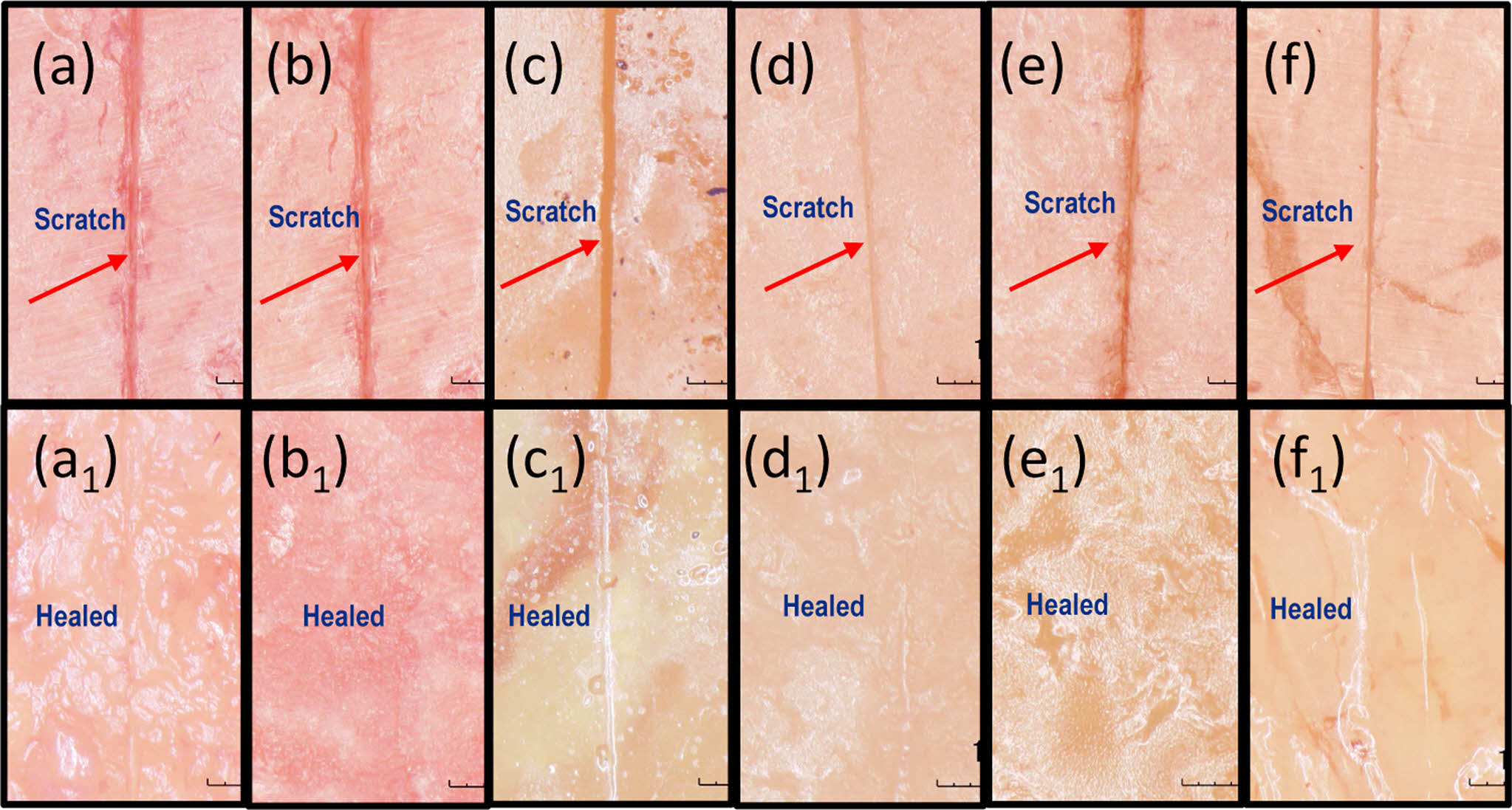
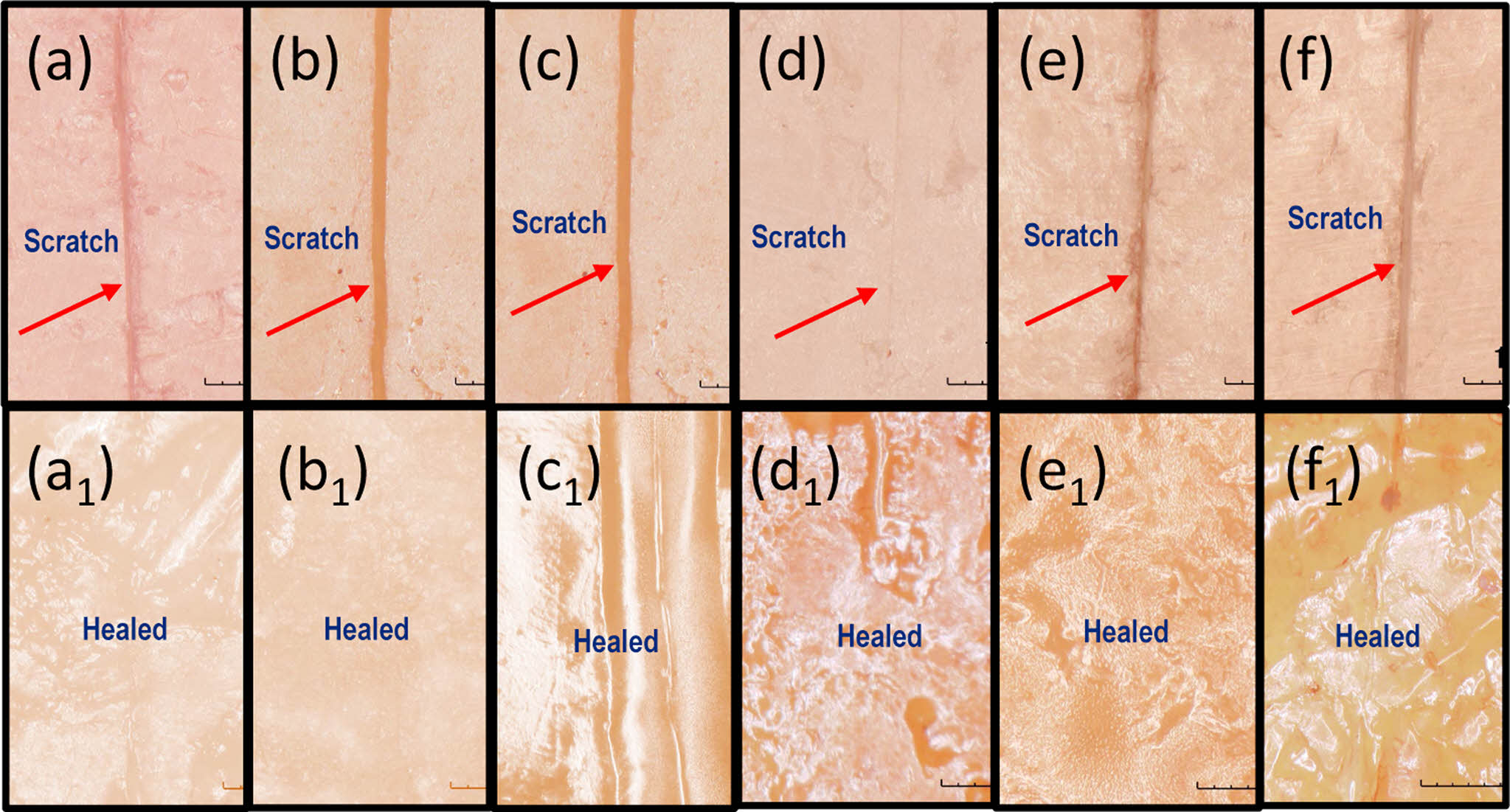
The self-healing property of polymers is a vital attribute, as it allows materials to repair themselves, thereby improving their longevity and performance in various applications. In this study, the self-healing characteristics of PU-DA samples formulated with both catalysts were systematically investigated using qualitative and quantitative methods. The qualitative analysis was performed by observing scratch recovery on PU-DA films after thermal treatment at 130 ℃ for 10 minutes, whereas the quantitative evaluation involved measuring the extent of tensile strength recovery following the healing process. Figure 7 shows the response of the PU-DA film samples to heating at 130 ℃. During heating, the scratched surfaces began to close, and the separated parts returned to their original positions. However, some scratches remained partially open, indicating partial healing.35 In contrast, scratches on PU-DA films heated at 130 ℃ for 2 hours disappeared completely (Figure 8). Thermal activation facilitates polymer chain mobility, enabling the damaged regions to undergo effective self-repair. Upon subsequent cooling to 70 ℃, the reformation of DA linkages are reformed, thereby reinstating the cross-linked network structure.36 Temperature significantly affects chain mobility, where elevated thermal conditions activate the retro-DA reaction, thereby enhancing the interfacial diffusion of polymer segments and promoting efficient crack closure.
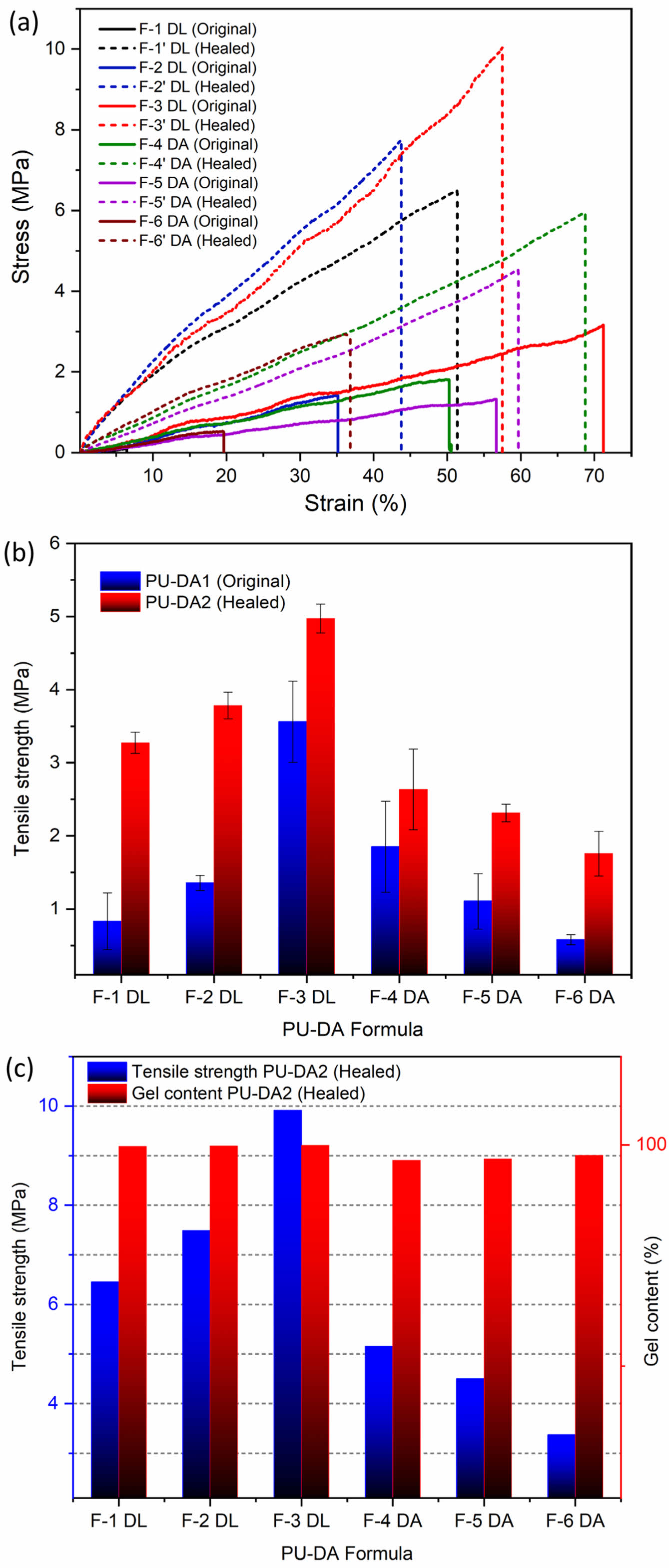
The self-healing capability of PU-DA synthesized with both catalysts was quantitatively evaluated by measuring the pre-cracked samples tensile properties original and healed samples. The analysis of the tensile test data focused on comparing the impact of each catalyst type, enabling an assessment of their respective contributions to the recovery of mechanical strength. As shown in Figure 9 and Table S2, the healed specimens tensile strength increased for both catalyst systems compared to the unhealed state. PU-DA samples prepared with the DBTDL catalyst exhibited significantly greater tensile strength enhancements than those synthesized with DBTDA. This improvement is primarily ascribed to the higher Lewis acidity of DBTDL, its enhanced compatibility with the polymer matrix—owing to its extended alkyl chains—and its superior catalytic efficiency. These factors collectively facilitate more effective urethane cross-linking, resulting in increased chain mobility and improved self-healing performance.37,38
This observation is supported by gel content data (Figure 9(c)), which reveal that PU-DA samples containing DBTDL possess a higher degree of crosslinking than those synthesized with DBTDA. Moreover, the healing efficiencies of both systems exceeded 100%, which is likely attributable to increased crosslink density during the healing process, resulting in post-healing tensile strengths surpassing those of the original, untreated samples. These findings suggest that enhanced gel content contributes to improved mechanical performance by promoting DA linkage reformation within the polymer matrix.39,40 The observed self-healing performance is closely related to the diffusion ability of polymer chains within the damaged region and the reformation of DA bonds via the broken crosslink reversible reaction.
|
Figure 1 Reaction pathway for the synthesis of self-healing PUs via the DA reaction. |
|
Figure 2 FTIR spectra of (a) PU-DA synthesized with different catalysts; (b) the corresponding zoomed-in spectra in the 2000– 1550 cm-1, wavenumber range. |
|
Figure 3 1 H NMR spectra of PU-DA using (a) DBTDA; (b) DBTDL as catalysts. |
|
Figure 4 DSC profiles of the original PU-DA samples with DBTDL and DBTDA catalysts during (a) the first heating cycle; (b) the cooling cycle; (c) the second heating cycle. |
|
Figure 5 (a) Density; (b) gel content of self-healing PU original and healed recovery using DBTDL and DBTDA catalysts. |
|
Figure 6 (a) Sequence shows the thermal treatment protocol applied to the PU-DA samples, while the DSC curves during the second heating cycle are shown (b) for the original, untreated PU-DA (PUDA1) in; (c) for the thermally healed PU-DA (PU-DA2) in. Note: PU-DA1 represents the original DA bond, r-PU-DA1 indicates the broken DA bond, and PU-DA2 illustrates the re-formed DA bond. |
|
Figure 7 Images obtained using a digital microscope show self-healing PU films catalyzed by DBTDL and DBTDA after being scratched and subsequently heated at 130 ℃ for 10 minutes. Images (a)~(f) correspond to the initial scratched surfaces of samples F-1 DL, F-2 DL, F-3 DL, F-4 DA, F-5 DA, and F-6 DA, respectively. The corresponding healed surfaces are shown in images a1, b1, c1, d1, e1, and f1. |
|
Figure 8 Images captured using a digital microscope of self-healing PU catalyzed by DBTDL and DBTDA after being scratched and subjected to a two-step thermal treatment—heating at 130 ℃ for 2 hours followed by holding at 70 ℃ for 24 hours. Images (a)~(f) show the scratched surfaces of samples F-1 DL, F-2 DL, F-3 DL, F-4 DA, F-5 DA, and F-6 DA, respectively, while images a1 to f1 illustrate the corresponding surfaces after the healing process. |
|
Figure 9 Impact of the healing process on the mechanical characteristics of PU-DA samples, illustrated by (a) stress-strain curves; (b) tensile strength; (c) gel content-tensile strength correlation. |
PUs with self-healing properties, synthesized through the DA reaction, were successfully developed using both DBTDL and DBTDA catalysts, each following a similar reaction mechanism. FTIR analysis showed that both catalysts effectively facilitated the formation of urethane and furan-maleimide bonds, with no significant differences observed in the chemical reaction. However, DSC analysis revealed that the DBTDL catalyst led to a higher degree of DA crosslinking, as indicated by a more significant decrease in PEG melting enthalpy and a higher gel content. Visual inspection using a digital microscope and tensile strength testing provided two complementary approaches for evaluating the self-healing ability of the PU in repairing damage and restoring mechanical strength. Tensile testing demonstrated that both catalysts achieved self-healing efficiencies exceeding 100%, with DBTDL showing better tensile strength recovery. Additionally, DSC results confirmed the thermal reversibility of DA bonds via the retro-DA reaction, enabling the bonds to reform upon heating. Overall, the study highlights that DBTDL is more effective in enhancing DA crosslink formation and polymer network recovery, improving the self-healing performance of bio-based PUs. The findings underscore the importance of selecting the right catalyst to optimize the mechanical properties and self-healing effectiveness of materials, particularly in the context of sustainable coating applications.
- 1. Haponiuk, J. T. Polyurethane Polymers, Blends and Interpenetrating Polymer Networks. In Polyurethane Polymers; Sabu. Thomas, Janusz. Datta, Jozef. T. Haponiuk, A. R., Ed.; Elsevier: Amsterdam, 2017; pp i-iii.
-

- 2. Akindoyo, J. O.; Beg, M. D. H.; Ghazali, S.; Islam, M. R.; Jeyaratnam, N.; Yuvaraj, A. R. Polyurethane Types, Synthesis and Applications-a Review. RSC Adv. 2016, 6, 114453-114482.
-

- 3. Liu, H.; Yang, P.; Li, Z.; Wen, Q.; Li, X.; Zhu, C.; Jiao, P.; Zhuang, W.; Wu, J.; Ying, H. Thermodynamics, Characterization, and Polymorphic Transformation of 1,5-Pentanediamine Carbonate. Ind. Eng. Chem. Res. 2020, 59, 10185-10194.
- 4. Willocq, B.; Odent, J.; Dubois, P.; Raquez, J. M. Advances in Intrinsic Self-healing Polyurethanes and Related Composites. RSC Adv. 2020, 10, 13766-13782.
-

- 5. Wang, D.; Chen, S.; Zhao, J.; Zhang, Z. Synthesis and Characterization of Self-healing Cross-linked Non-isocyanate Polyurethanes Based on Diels-Alder Reaction with Unsaturated Polyester. Mater. Today Commun. 2020, 23, 101138.
-

- 6. Sriharshitha, S.; Krishnadevi, K.; Devaraju, S.; Srinivasadesikan, V.; Lee, S.-L. Eco-Friendly Sustainable Poly(benzoxazine-co-urethane) with Room-Temperature-Assisted Self-Healing Based on Supramolecular Interactions. ACS Omega 2020, 5, 33178-33185.
-

- 7. Zhu, M.; Liu, J.; Gan, L.; Long, M. Research Progress in Bio-based Self-healing Materials. Eur. Polym. J. 2020, 129.
- 8. Tremblay-Parrado, K.-K.; Avérous, L. Renewable Responsive Systems Based on Original Click and Polyurethane Cross-Linked Architectures with Advanced Properties. ChemSusChem 2020, 13, 238-251.
-

- 9. Li, W.; Li, H.; Wu, C.; Han, B.; Ouyang, P.; Chen, K. An Effective Synthesis of Bio-based Pentamethylene Diisocyanate in a Jet Loop Reactor. Chem. Eng. J. 2021, 425.
- 10. He, F.; Tang, Y.; Lu, Z.; Hu, Q.; Yang, Y.; Li, G.; Li, H.; Chen, K. An Effective Purification of Double-effect Distillation for Bio-based Pentamethylene Diisocyanate. RSC Adv. 2023, 13, 31518-31527.
-

- 11. Tawade, B. V; Shingte, R. D.; Kuhire, S. S.; Sadavarte, N. V; Garg, K.; Maher, D. M.; Ichake, A. B.; More, A. S.; Wadgaonkar, P. P. Bio-Based Di-/Poly-isocyanates for Polyurethanes: An Overview. PU Today 2017, 41-46.
- 12. Lenzi, V.; Crema, A.; Pyrlin, S.; Marques, L. Current State and Perspectives of Simulation and Modeling of Aliphatic Isocyanates and Polyisocyanates. Polym. 2022, 14, 1642.
-

- 13. Zhang, J.; Tu, W.; Dai, Z. Synthesis and Characterization of Transparent and High Impact Resistance Polyurethane Coatings Based on Polyester Polyols and Isocyanate Trimers. Prog. Org. Coatings 2012, 75, 579-583.
-

- 14. Wang, H.; Cao, L.; Wang, X.; Lang, X.; Cong, W.; Han, L.; Zhang, H.; Zhou, H.; Sun, J.; Zong, C. Effects of Isocyanate Structure on the Properties of Polyurethane: Synthesis, Performance, and Self-Healing Characteristics. Polym. 2024, 16, 3045.
- 15. Yu, C.; Wang, Z.; Fei, G.; Zhang, X.; de Luna, M. S.; Lavorgna, M.; Xia, H. Robust Self-healing Waterborne Polyurethane Coatings via Dynamic Covalent Diels-Alder Bonds for Corrosion Protection. J. Polym. Sci. 2024, 62, 815-825.
-

- 16. Wei, Y.; Ma, X. The Self-healing Cross-linked Polyurethane by Diels–Alder Polymerization. Adv. Polym. Technol. 2018, 37, 1987-1993.
-

- 17. Sridhar, L. M.; Oster, M. O.; Herr, D. E.; Gregg, J. B. D.; Wilson, J. A.; Slark, A. T. Re-usable Thermally Reversible Crosslinked Adhesives from Robust Polyester and Poly(ester urethane) Diels-Alder Networks. Green Chem. 2020, 22, 8669-8679.
-

- 18. Li, Q.; He, H.; Ye, X.; Guan, F.; Ai, Y.; Shen, Y.; Zhang, C. NIR Light-induced Functionalized MXene as a Dynamic-crosslinker for Reinforced Polyurethane Composites with Shape Memory and Self-healing. Chem. Eng. J. 2023, 475, 146500.
-

- 19. Huang, X.; Wang, X.; Shi, C.; Liu, Y.; Wei, Y. Research on Synthesis and Self-healing Properties of Interpenetrating Network Hydrogels Based on Reversible Covalent and Reversible Non-covalent Bonds. J. Polym. Res. 2021, 28, 1.
-

- 20. Hu, C.; Li, J.; Pan, X.; Zeng, Y. Intrinsically Flame-retardant Vanillin-based PU Networks with Self-healing and Reprocessing Performances. Ind. Crops Prod. 2023, 200, 116828.
-

- 21. Sung, S.; Kim, S. Y.; Lee, T. H.; Favaro, G.; Park, Y. I.; Lee, S.-H.; Ahn, J. B.; Noh, S. M.; Kim, J. C. Thermally Reversible Polymer Networks for Scratch Resistance and Scratch Healing in Automotive Clear Coats. Prog. Org. Coatings 2019, 127, 37-44.
-

- 22. Irusta, L.; Fernández-Berridi, M. J.; Aizpurua, J. Polyurethanes Based on Isophorone Diisocyanate Trimer and Polypropylene Glycol Crosslinked by Thermal Reversible Diels Alder Reactions. J. Appl. Polym. Sci. 2017, 134, DOI: 10.1002/app.44543.
-

- 23. Aizpurua, J.; Martin, L.; Formoso, E.; González, A.; Irusta, L. One Pot Stimuli-responsive Linear Waterborne Polyurethanes via Diels-Alder Reaction. Prog. Org. Coatings 2019, 130, 31-43.
-

- 24. Kim, H.-N.; Lee, D.-W.; Ryu, H.; Song, G.-S.; Lee, D.-S. Preparation and Characterization of Isosorbide-based Self-healable Polyurethane Elastomers with Thermally Reversible Bonds. Molecules 2019, 24, 1061.
-

- 25. Varganici, C.-D.; Ursache, O.; Gaina, C.; Gaina, V.; Rosu, D.; Simionescu, B. C. Synthesis and Characterization of a New Thermoreversible Polyurethane Network. Ind. Eng. Chem. Res. 2013, 52, 5287-5295.
-

- 26. Gaina, C.; Ursache, O.; Gaina, V.; Varganici, C. D. Thermally Reversible Cross-linked Poly(ether-urethane)s. Express Polym. Lett. 2013, 7, 636-650.
-

- 27. Lakatos, C.; Czifrak, K.; Karger-Kocsis, J.; Daroczi, L.; Zsuga, M.; Keki, S. Shape Memory Crosslinked Polyurethanes Containing Thermoreversible Diels-Alder Couplings. J. Appl. Polym. Sci. 2016, 133, DOI: 10.1002/app.44145.
-

- 28. Behera, P. K.; Raut, S. K.; Mondal, P.; Sarkar, S.; Singha, N. K. Self-Healable Polyurethane Elastomer Based on Dual Dynamic Covalent Chemistry Using Diels-Alder ‘Click’ and Disulfide Metathesis Reactions. ACS Appl. Polym. Mater. 2021, 3, 847-856.
-

- 29. Fu, G.; Yuan, L.; Liang, G.; Gu, A. Heat-resistant Polyurethane Films with Great Electrostatic Dissipation Capacity and Very High Thermally Reversible Self-healing Efficiency Based on Multi-furan and Liquid Multi-maleimide Polymers. J. Mater. Chem. A 2016, 4, 4232-4241.
-

- 30. Du, P.; Wu, M.; Liu, X.; Zheng, Z.; Wang, X.; Joncheray, T.; Zhang, Y. Diels-Alder-based Crosslinked Self-healing Polyurethane/urea from Polymeric Methylene Diphenyl Diisocyanate. J. Appl. Polym. Sci. 2014, 131, DOI: 10.1002/app.40234.
-

- 31. Li, Y.; Yang, Z.; Zhao, X.; Zhang, J.; Ding, L.; Pan, L.; Lin, C.; Zheng, X. Practicable Self-healing Polyurethane Binder for Energetic Composites Combining Thermo-reversible DA Action and Shape-memory Effect. Polym. Adv. Technol. 2021, 32, 4223-4232.
-

- 32. Du, X.; Jin, L.; Deng, S.; Zhou, M.; Du, Z.; Cheng, X.; Wang, H. Recyclable, Self-Healing, and Flame-Retardant Solid-Solid Phase Change Materials Based on Thermally Reversible Cross-Links for Sustainable Thermal Energy Storage. ACS Appl. Mater. Interfaces 2021, 13, 42991-43001.
-

- 33. Li, J.; Zhang, G.; Deng, L.; Zhao, S.; Gao, Y.; Jiang, K.; Sun, R.; Wong, C. In Situ Polymerization of Mechanically Reinforced, Thermally Healable Graphene Oxide/polyurethane Composites Based on Diels-Alder Chemistry. J. Mater. Chem. A 2014, 2, 20642-20649.
-

- 34. Wang, Z.; Zhou, J.; Liang, H.; Ye, S.; Zou, J.; Yang, H. A Novel Polyurethane Elastomer with Super Mechanical Strength and Excellent Self-healing Performance of Wide Scratches. Prog. Org. Coatings 2020, 149, 105943.
-

- 35. Xu, Y.; Chen, D. Shape Memory-assisted Self-healing Polyurethane Inspired by a Suture Technique. J. Mater. Sci. 2018, 53, 10582-10592.
-

- 36. Zheng, K.; Tian, Y.; Fan, M.; Zhang, J.; Cheng, J. Recyclable, Shape-memory, and Self-healing Soy Oil-based Polyurethane Crosslinked by a Thermoreversible Diels–Alder Reaction. J. Appl. Polym. Sci. 2018, 135, DOI: 10.1002/app.46049.
-

- 37. Mashlyakovskiy, L.; Khomko, E.; Volynkina, N.; Tonelli, C. Fluoropolyethers End‐capped by Polar Functional Groups. III. Kinetics of the Reactions of Hydroxy‐terminated Fluoropolyethers and Model Fluorinated Alcohols with Cyclohexyl Isocyanate Catalyzed by Organotin Compounds. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 3771-3795.
-

- 38. Renault, B.; Cloutet, E.; Cramail, H.; Hannachi, Y.; Tassaing, T. A Combined Spectroscopic and Theoretical Study of Dibutyltin Diacetate and Dilaurate in Supercritical CO2. J. Phys. Chem. A 2008, 112, 8379-8386.
-

- 39. Sirajuddin, N. A., Jamil, M. S. M. & Lazim, M. A. S. M. Effect of Cross-link Density and the Healing Efficiency of Self-healing Poly(2-hydroxyethyl methacrylate) Hydrogel. E-Polymers 2014, 14, 289-294.
-

- 40. Boden, J.; Bowen, C. R.; Buchard, A.; Davidson, M. G.; Norris, C. Understanding the Effects of Cross-Linking Density on the Self-Healing Performance of Epoxidized Natural Rubber and Natural Rubber. ACS Omega 2022, 7, 15098-15105.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2024 Impact Factor : 0.6
- Indexed in SCIE
 This Article
This Article
-
2025; 49(6): 818-828
Published online Nov 25, 2025
- 10.7317/pk.2025.49.6.818
- Received on Jun 26, 2025
- Revised on Jul 30, 2025
- Accepted on Aug 1, 2025
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
- Conflict of Interest
- Supporting Information
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Heru Santoso*, **, Sumarno Sumarno*
-
*Department of Chemical Engineering, Institut Teknologi Sepuluh Nopember (ITS), Surabaya 60111, Indonesia
**Research Center for Polymer Technology, National Research and Innovation Agency (BRIN), KST BJ. Habibie 460 building, South Tangerang 15314, Indonesia - E-mail: heru011@brin.go.id, onramus@chem-eng.its.ac.id
- ORCID:
0009-0001-3669-3350, 0000-0003-1137-5054








 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.