- Polyhydroxyalkanoate Production from Food Waste Using Bacillus megaterium
Humera Arshad# , Farrukh Jamil# , Rana Umer Hayat, Muhammad Qaisar Hafeez, Amna Kousar, Muhammad Ibrahim, and Muhammad Asif Rasheed†

Department of Biosciences, COMSATS University Islamabad, Sahiwal Campus, Sahiwal 57000, Pakistan
- Bacillus megaterium를 활용한 음식물 쓰레기에서 Polyhydroxyalkanoate 제조
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
Polyhydroxyalkanoates (PHA) emerged as a promising material with similar physical properties as petroleum based plastic and it is biodegradable. Several microorganisms synthesize and accumulate this material under specific conditions. A strain of Bacillus megaterium (BM2) was used to produce PHA by using wasted rice, potato, fruit, cheese whey, molasses and frying oil. Since molasses, hydrolyzed rice media and frying oil produced higher yield compared to the other wasted materials so they were further optimized at various concentrations. The highest PHA yield obtained (57.33% and 53.92%) by cell mass with hydrolyzed wasted rice media and 2% molasses in Nutrient broth medium respectively whereas 56.89% PHA yield was obtained with wasted frying oil supplemented medium and wasted potato medium as well.
PHA production by using several wasted food source like wasted rice, potato, fruit, cheese whey molasses and frying oil. The yield of PHA using 40% hydrolyzed wasted potato media and wasted rice media supplemented with 60% nutritional broth media were 0.6 g/100 mL and 0.86 g/100 mL (40% and 57.33%) respectively.
Keywords: bioplastic, biodegradable, food waste, microbes, PHA, wasted frying oil.
We acknowledge Higher Education Commission, Islamabad, Pakistan (HEC) for funding this study under the NRPU project number 20-9335/R&D/NRPU/HEC/2018.
The authors declare that there is no conflict of interest.
Plastic has replaced several metal-based materials in our daily life due to its unique physical properties such as light weight, durability and low conductivity.1 Several plastic-based utensils, containing tons of plastic, are prepared every year around the globe. On the other hand, a significant amount of plastic waste is produced annually.2-4 Such an enormous amount of plastic waste, which is not degradable, has created serious concern for our environment.5,6 Additionally, it contaminates our air with toxic gases when burnt, and our water resources. Therefore, it is the need of the time to find a substitute of environmentally friendly plastic.7
Polyhydroxyalkanoates (PHA) a biodegradable microbial bio-polyester which is synthesized and accumulated by certain microorganisms under low nutrient conditions such as nitrogen, oxygen and phosphorous.8-10 The polymers host some considerably similar physical properties as that of petroleum-based plastics and it is biodegradable by certain soil bacteria.4,6,9-11 Therefore, it has the potential to replace petroleum-derived plastic.4,7,12-15 The most promising feature of PHA is its biocompatibility.14,16 Therefore, several medical devices have been reported based on PHA such as bone marrow scaffolds, cardiovascular patches, wound dressings and tendon repair devices.9,15,17-21
PHA has been classified into three different classes: small, medium and long-chain on the basis of the number of carbon atoms in the polymer. Small chain length polymer contains 3 to 6 carbon atoms and medium-chain includes 6 to 14 carbon atoms whereas long polymer host more than 14 carbon atoms.22,23 Studies have highlighted that the physical properties of different types of PHA vary with their chain length.15,24-26
Researchers worldwide are trying to reduce cost of PHA production by using different strategies as high cost is a limiting factor for its commercial level production. Industrial level production of PHA range 2.2-3.3€ per kg almost to 2 to 3 fold higher than petroleum based plastic.27 For this purpose scientist are trying to use waste carbon sources as a feedstock for the microorganisms and using cheap PHA isolation and purification methods.28-31
Studies have shown that several different waste materials can be used as a carbon sources for the PHA production by using PHA accumulating microorganism, such as sludge, industry effluent and wasted food materials.15,24-26,32 PHA accumulating microorganisms convert waste carbon into PHA and store them as a food source. Therefore, using waste carbon sources will reduce the cost of production.9,10,15,20,21,24-26,33,34
This study aims to isolate and identify local PHA accumulating microbes from soil, water and dumping sites of food waste from different cities in the Punjab province of Pakistan. Moreover, it aims to use these PHA accumulating bacteria to convert the several different food waste materials into PHA. On the other hand, in future we will also try to reduce the production cost by using alternate methods for PHA isolation and purification.
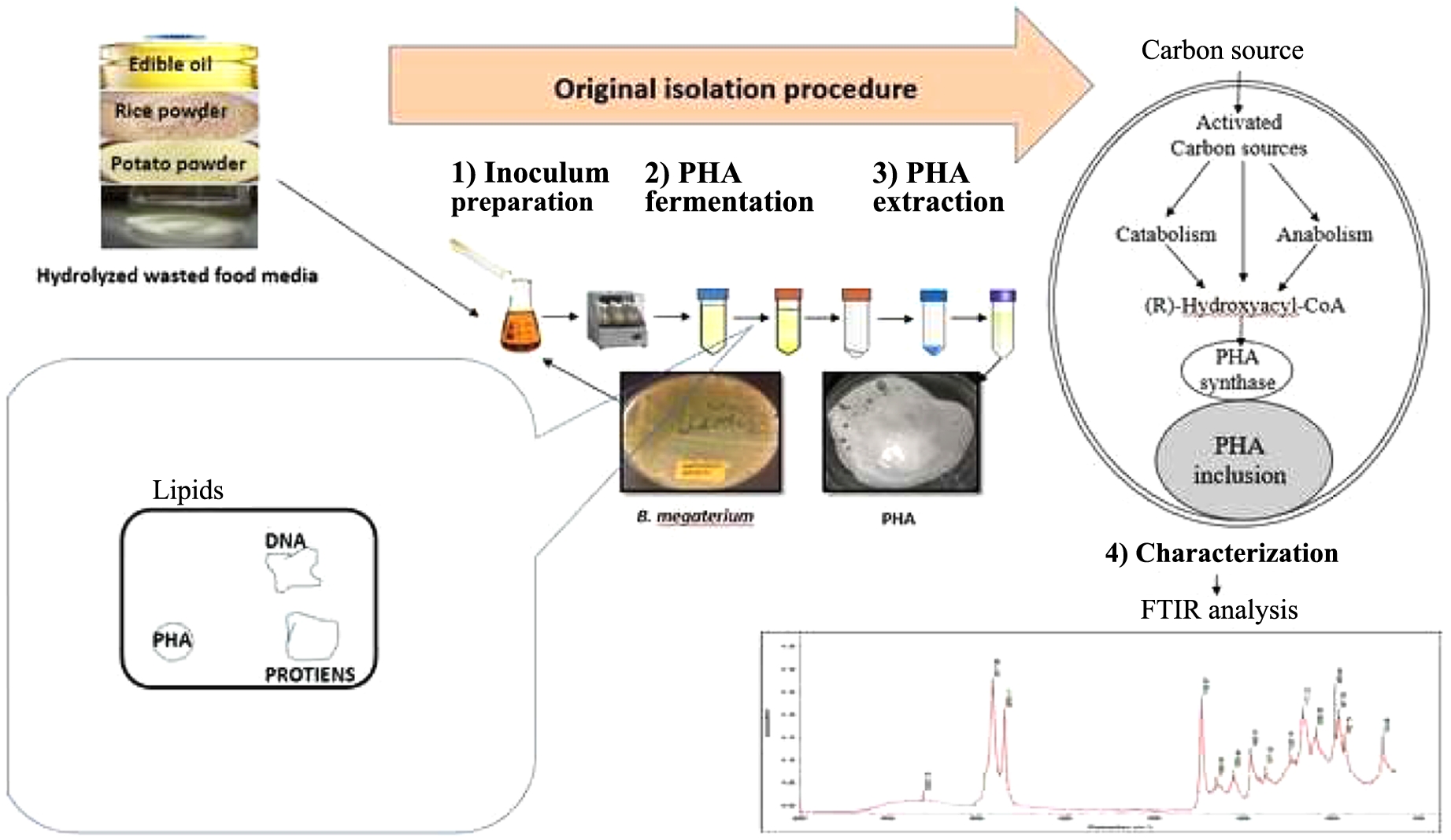
Sample Collection. Several different samples of soil, water, fruits, vegetables, used cooking oil, sugarcane bagasse, poultry waste and cheese whey were collected from different cities of the Punjab. The soil samples were collected 5 to 10 cm deep from the surface and preserved for bacterial isolation, whereas wasted fruit/vegetable, sugarcane husk, poultry waste and cheese whey samples were collected in small amounts and preserved in sterile falcon tubes.
Sample Spreading on Agar Plates. Luria-Bertani (LB) agar medium was supplemented with 2% glucose (Sigma Aldrich, USA) and 0.5 μg/mL Nile Blue A(Applichem, Germany) was used for agar plate preparation. This method allowed us to screen PHA producing bacteria as they show orange/pink fluorescence under UV irradiation.35 The samples were suspend in distilled autoclaved water in 1 to 10% W/V and 10 μL were spread on Nile Blue supplemented agar plates.
Screening of PHA Accumulating Colonies. Colonies obtained after incubation were screened under UV light. PHA producing colonies showed light orange or light pink fluorescence under UV irradiation. Colonies were marked and preserved as glycerol (Sigma Aldrich, USA) stock. Moreover, only separate colonies were used for genomic DNA isolation, identification and small scale PHA production test.
Genomic DNA Isolation. Well separated pink/orange colonies were grown in 5 mL LB broth media and allowed to grow for 24 h at 37 ℃ at 100 rpm in an orbital shaker (Biobase, China). After 24 h, it was transferred into another flask containing 20 mL LB broth media (1% inoculum) and allowed to grow at 37 ℃ at 100 rpm in an orbital shaker. Cells harvested by centrifugation at standard conditions. Pellet washed with autoclaved water and centrifuged for pellet formation. For genomic DNA isolation standard protocol was used as described by Sambrook and Russell, 2001.36 The quality of DNA was analyzed on 1% agarose (Sigma Aldrich, USA) gel.
Molecular Identification of PHA Producing Colonies. Bacterial 16S rRNA gene was amplified by using universal 27F(5′-AGAGTTTGATCATGGCTCAG-3′)as forward primer and 1492R(5′-GGTTACCTTGTTACGACTT-3′) as reverse primer,and using the isolated DNA sample as template with annealing temperature of 55 ℃. The PCR products were fractionated on agarose gel and purified from the gel by gel extract kit (Thermo Scientific, USA). The purified PCR product was sequenced by using automatic DNA sequencer (Apical Scientific, Malaysia).
Small Scale PHA Production and Extraction from Screened Colonies. A single colony was grown in 5 mL Minimal salt medium media (MSM) supplemented with 2% glucose (Sigma Aldrich, USA) at 37 ℃ and kept on shaking at 100 rpm in an orbital shaker for 24 h.37,38 It was inoculated (1%) into another flask containing 100 mL of same media and grown for 48 h at 37 ℃ and 100 rpm then cells were harvested. PHA was precipitated from the halogenated solvent by using chilled methanol (Riedel-de Haen, Germany) and formation of white precipitate was observed in PHA accumulating colonies.
Food Waste Supplemented MSM and Nutrient Broth Medium. Later, MSM and Nutrient Broth medium (NB) containing 0.05% (NH4)2SO4 (Sigma Aldrich, USA) and 1% glucose (Sigma Aldrich, USA) was used with different pre-digested food waste materials for PHA production.39 Three replicates of all the experiments were performed to get a reliable mean value.
Pre-treatment of Wasted Food. Wasted rice and potato samples were collected from different areas of Punjab, Pakistan. The samples were washed and dried. After drying the samples were minced and both the samples were treated with α-amylase (Sigma Aldrich, USA) (10 units) in 100 mM tris HCl buffer (Sigma Aldrich, USA) with pH 7.5 separately. The samples were kept for 48 h in orbital shaker (Biobase, China) at 120 rpm and 37 ℃.
Waste frying oil and juice were collected from different local vendors. Molasses was obtained from local market. 1 Kg fruit pulp was kept in 500 mL water for 24 h. All these pre-treated samples were used as supplement in MSM and NB medium at 1 to 5% concentration.
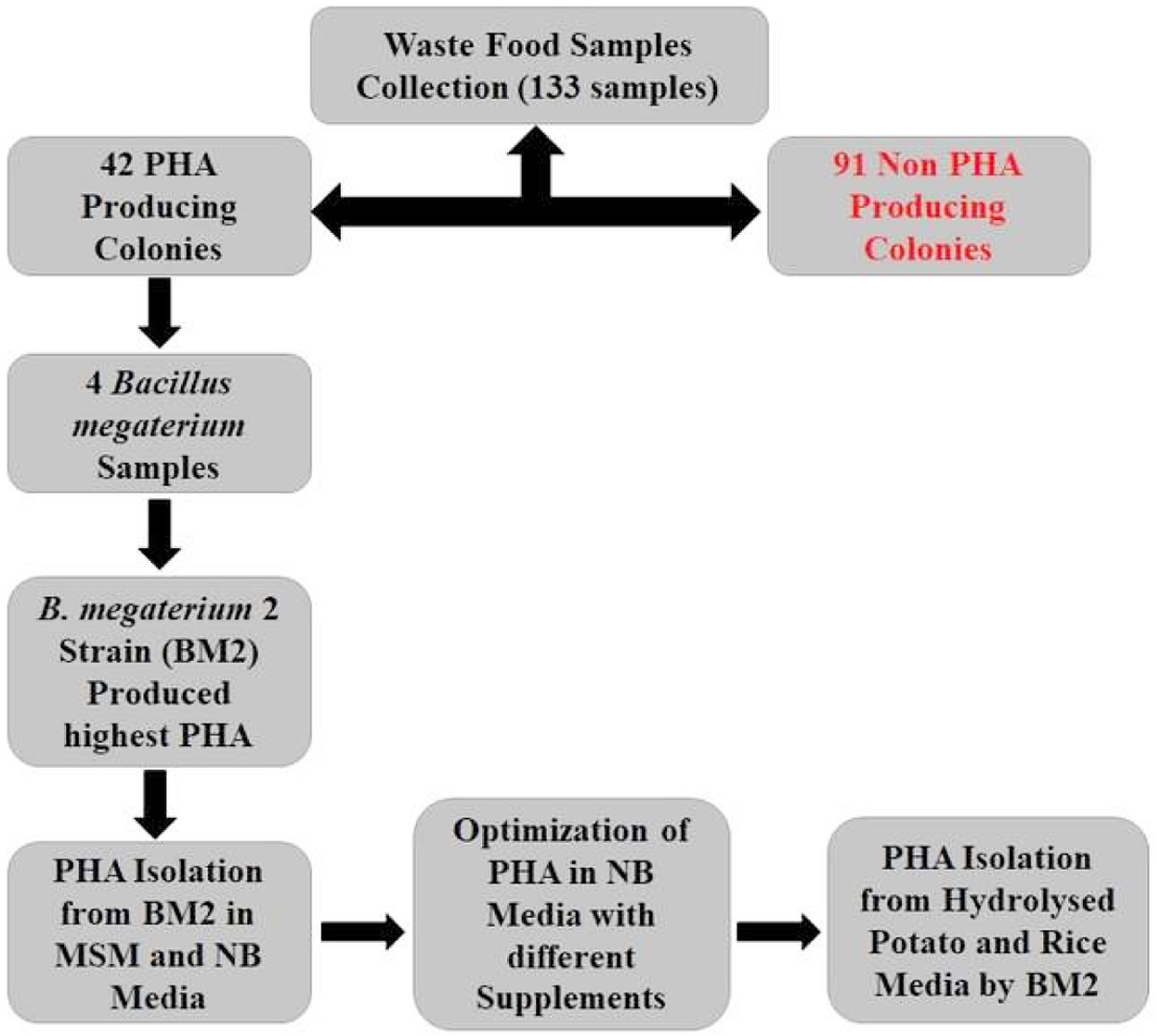
Screening of PHA accumulating colonies from all collected sample showed that forty-two samples have different numbers of pink/orange colonies under UV in the presence of Nile Blue A (NBA) whereas 91 samples showed negative results. The detail of the workflow is shown in Figure 1. PHA accumulating colonies give orange or pink color under UV.34 Positive colonies screened with Sudan Black B (SB) and all of them showed positive results. Studies have shown that SB dye may bind to the intracellular granules specifically to its lipid part.35 So far several PHA accumulating microorganisms have been screened by using this method from paper pulp, cheese whey, soil and water samples.4,6,9-11
The positive colonies were used for 16S rRNA gene amplification after genomic DNA isolation by using universal primers for 16S rRNA gene. Sequence of the 16S rRNA was obtained. BLAST analysis showed the sequence identity of the strains with already known strains. Data analysis highlighted that most of the strains are of Bacillus comprising B. cereus, B. pumulis, B. BA-126 and B. megaterium at the identity 92, 83, 84, and 95% respectively against 16S rRNA gene from already known strains. Moreover, we have also identified few strains of Enterobacter, Citrobacter, Halomonas and Klebsiella. Several previous studies have shown that the identified microbes can accumulate PHA at different conditions.40,41
Four strains of Bacillus megaterium were used for PHA production in 2% glucose supplemented in MSM. PHA was isolated after 48 h of growth from the four strains. 32, 41, 25, and 28% PHA yield was observed from strain 1, 2, 3 and 4 respectively. Therefore, strain 2; designated BM2 was used for further studies. A time course study was conducted by using, BM2 in 2% glucose supplemented in MSM. Analysis showed that PHA yield has increased with the increase in OD 600 nm but it decreases after 48 h while the growth enters in stationary phase. The effect of pH on the growth and PHA production revealed optimal pH 7 with highest yield (33%). On the other hand, PHA production reduced with the rise in pH and dropped to less than 5% at pH 10.
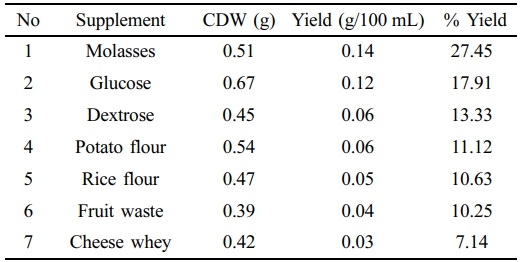
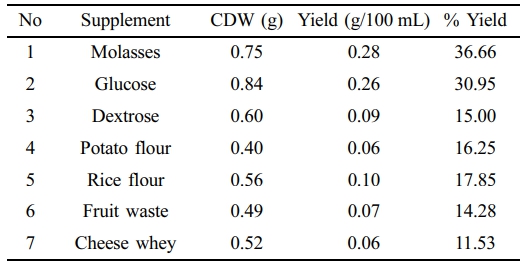
BM2 grown in MSM supplemented with several carbon sources like molasses, glucose, dextrose, potato flour, rice flour, fruit waste and cheese whey and 27.45, 17.91, 13.33, 11.12, 10.63, 10.25, and 7.14% PHA yields were obtained respectively by chloroform extraction method after 48 h at pH 7.0. On the other hand, 36.66, 30.95, 15, 16.25, 17.85, 14.28, and 11.53% PHA yields were observed in NB supplement with 2% of molasses, glucose, dextrose, potato flour, rice flour, fruit waste and cheese whey respectively (Table 1, 2).
Comparison of the two medium showed that NB supplemented with different supplements have produced higher yields compared to those in MSM.
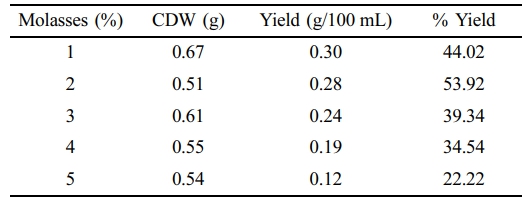
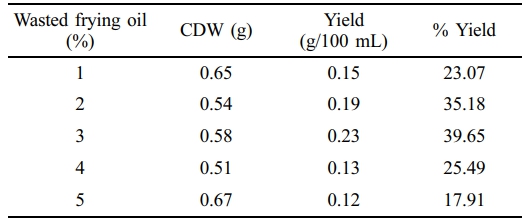
NB was supplemented with various concentrations of molasses (1-5%) at pH 7. The BM2 produced 44, 53.92, 39.34, 34.54, and 22.22% PHA at 1 to 5% supplemented molasses in NB, suggesting that 2% molasses as a supplement can produce more PHA compared to the higher concentrations (Table 3). Table 4 summarizes the PHA yield when NB was supplemented with wasted frying oil. Analysis of the data showed that 3% fatty acid can produce highest yield (39.65%) and higher concentrations decreased the PHA accumulation.
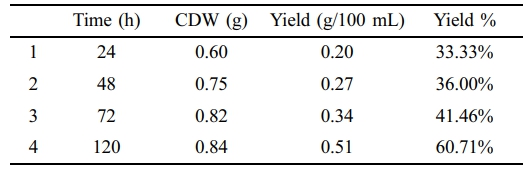
PHA extraction was also carried out for different time intervals such as 24, 48, 72, and 120 h using NB supplemented with food waste oil (100 µL). It gave 33.33%, 36.00%, 41.46%, and 60.71% yield at 24, 48, 72, and 120 h respectively (Table 5).
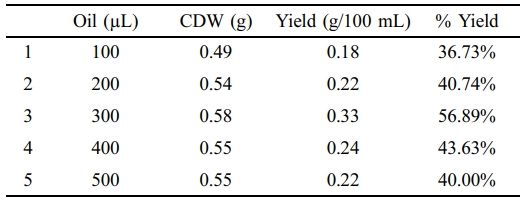
NB was supplemented by using different concentrations of wasted food oil from 100 µL to 500 µL. Among them, the highest yield of PHA was at 300 µL of waste edible oil, which was 56.89% (Table 6). It was observed that after increasing the concentration of waste food oil, the percentage yield also increased. After increasing 300 µL to 400 µL of oil, the percentage yield decreased.
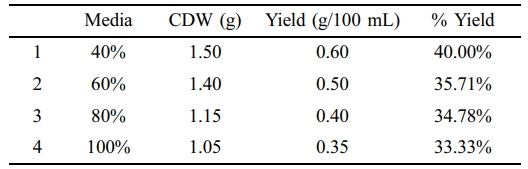
Pre hydrolyzed wasted potato was synthesized and used with NB (40, 60, 80, and 100%) supplemented with 300 µL of wasted edible oil. 40, 35.71, 34.78, and 33.33% PHA production were calculated at 40, 60, 80, and 100% of pre hydrolyzed media respectively. Calculations revealed minimal variation in percentage yields across various hydrolyzed medium and NB concentrations (Table 7).
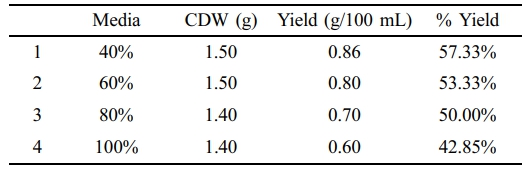
By using hydrolyzed cooked rice waste media in addition to hydrolyzed cooked potato waste media several experiments were performed. Additionally, hydrolyzed cooked rice waste medium at various percentage concentrations with NB (40%–100%) to obtain various PHA yields. Similar to hydrolyzed cooked potato waste media, computations using various hydrolyzed media and NB concentrations revealed minimal differences in percentage yields, as the table illustrates. 40% hydrolyzed cooked rice waste yielded 57.33% PHA (Table 8).
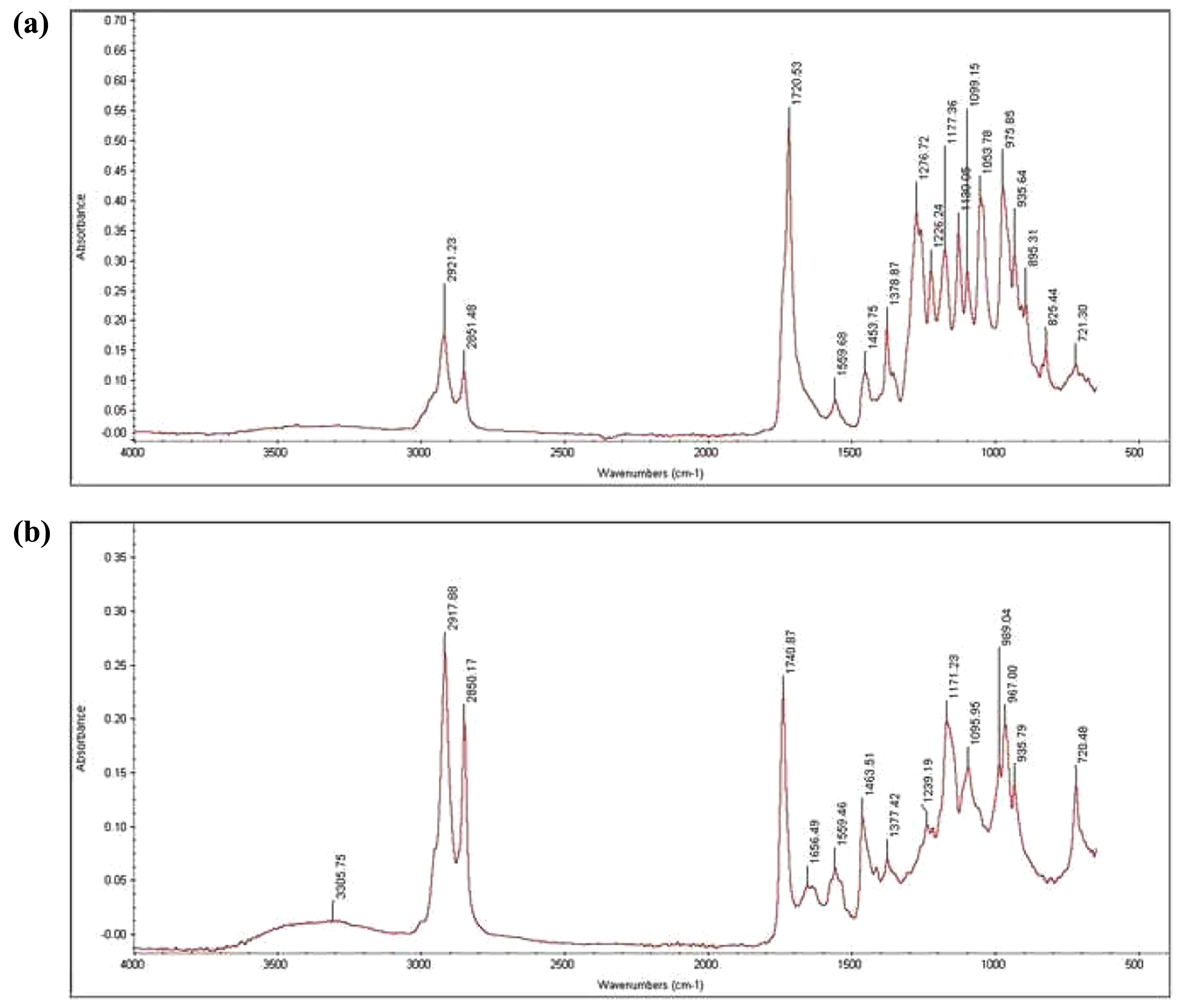
The FTIR analysis showed an intense peak at 1722 cm-1, which indicates the vibration of carbonyl (C═O) group of ester of PHA. Some stretches in range of 1330-1200 are due to -CH3, -CH2, -CH (Figure 2). Similar peaks have been reported in the previous several studies for PHA isolated from Bacillus species.42
Degradation experiments were performed under natural conditions in garden soil. Garden soil was poured into a clean glass jar. PHA specimens were weighed and buried along with the same-sized petro plastic (polyethylene) deep in the soil (Figure 3(a) and 3(b)). Specimens were observed at time intervals. Initially, the degradation of PHA was a slow process due to an environment with low microbial activity. Considerable degradation was observed in 2 weeks (Figure 3(c)) while major part was degraded after 30 days (Figure 3(d)).
Throughout the research season, the temperatures and humidity of the air, soil, and both study locations were comparable for both specimens. At the end of the incubation period, the degradation of the buried film reached 90%, and the film placed above the soil was 60% degraded. But there was no effect observed on petroleum derived plastic. The degradation rate of buried film was higher due to the availability of microbes and microbial activity that was higher deep in the soil from all directions. Because deep soil specimens had greater surface areas and bigger soil interface areas, which promoted better microbial adhesion and faster microbial degradation, it deteriorated more quickly than PHA film specimens above soil.
An organization of United Nation’s (Food and Agriculture Organization) showed that in 2019, a total of 14% of food was lost from harvest to consumptions due to poor transportation and lack of storage facilities. Food waste is a rich organic material containing lipids, cellulose, sugars and organic acids.43,44 Studies have shown that this valuable waste material can be converted into PHA with pure/mixed microbial culture.10,43,44 In case of mixed microbial culture simple sugars are not recommended and complex food wastes have been suggested.44
Pakistan is an agriculture-based country where almost 70% of people are directly or indirectly linked with agriculture.43 However, due to poor transport and storage facilities, considerable food is wasted every year in the country. On the other hand, almost 30 to 40% of cooked food is also wasted. Cooked and uncooked food can be used for PHA synthesis by using certain microorganisms.43,44 This will definitely reduce environment pollution by wasted plastic, which is getting worse day by day in Pakistan. In Pakistan, people use land to dispose this material. Even, most of the canals and rivers accumulate waste plastic bags, bottles and it is affecting the marine life. In order to reduce the cost of waste management, an alternate for plastic which can be degraded by microbial activity.
In this study wasted food specifically wasted rice, potato, fruit pulp, cheese whey, molasses and frying oil was used for PHA production by using the identified BM2. Rice and potato starch were hydrolyzed by using a-amylase and cheese whey was treated with protease prior to their use as carbon supplement. On the other hand, waste frying oil was used after lipase treatment. Studies have used these medium for PHA production with other B. megaterium strains.39,45 therefore it was used these as growth medium supplemented with predigested food waste.
The highest PHA yield was observed (around 53.92%) by using NB medium supplemented with 2% molasses and around 30% by using glucose. Tajima et al., 2003 has also reported a very close yield of PHA by using other B. megaterium strain in glucose supplement medium. Analysis of the data showed that NB is more suitable medium compare to the MSM medium if supplemented with the above mentioned waste food supplement (Tables 1-2).Studies have shown that the production of PHA by Alcaligenes eutrophus in a synthetic medium has produced highest yield at 3% glucose supplemented medium.
Highest yield of PHA was observed by using 3% fatty acid supplement in NB medium whereas molasses provided highest yield (53.90%) at 2% supplement in NB.46 Studieshave reported PHA production by using different wasted frying oil as carbon source for C. nector.47-49
The study has shown that time of incubation has a relation with yield. As by increased cell harvesting time the count of cell dry weight and yield also increased. PHA yielded best at 120 h that was 60.71% (Table 5). Studies have shown that neutral to alkaline pH also improves the yield of PHA as it is optimal pH for enzymes involves in its biosynthesis.50
Different percentages of PHA production were observed using different concentrations of food oil as supplement. PHA yield was increased with increased the concentration of food oil in growth media but at a 300 µL of food oil PHA yield was maximum after that PHA synthesis decreased (Table 6). It was observed that a specific concentration of oil is facilitating the growth media but in over saturation bacteria cannot grow. The highest yield of PHA was at 300 µL of waste food oil (56.89%).
Two types of cooked food waste hydrolyzed media that were from cooked potato waste and cooked rice waste were studied for PHA production. Different studies have shown that utilizing food waste as a culture medium would not only reduce the cost of the substrate needed to produce PHA but also ease the burden of waste management. 40% hydrolyzed wasted potato and wasted rice media yielded 0.6 g/100 mL (40%) and 0.86 g yield/100 mL (57.33%) PHA respectively.
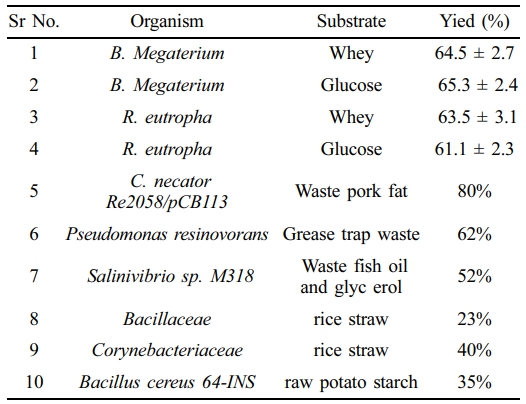
Studies have shown that different carbon sources have been used for the PHA production by using different microbes such as Bacillius megaterium, Cupriavidus necator, Bacillus cereus, Pseudomonas resinovorans and other microbes as mentioned in Table 9.51-56However, this study developed a medium based on the wasted potatoes and rice to prepare PHA in large amount. It has reduced the cost of PHA production remarkably and both the products are major crops of the Punjab, Pakistan.
The produced PHA with standard PHA from Sigma Aldrich. PHA was subjected to FTIR analysis to validate its composition. A prominent peak at 1722 cm-1 was identified by FTIR study as a distinctive property of PHA. Additionally, stretches between 1330-1200 cm-1 were found, corresponding to vibrations linked to the -CH3, -CH2, -CH groups (Figure 2). Notably, a great deal of prior research on PHA isolated from various species has consistently shown identical spectral peaks.57 Further studies are in progress to determine its physical and chemical features for specific applications.
The degradation pattern of PHA films can be influenced by an extensive range of environmental conditions, including temperature, microbial activity, pH, soil type, moisture content, and nutrient availability. In addition, the characteristics of the PHA, such as its surface area, crystallinity, composition, and additive presence, may also have an impact on how quickly it degrades.58 In this study garden soil was chosen to evaluate biodegradability of PHA films produced previously as explained in results.
This study was conducted for three months. The average mass reduction of the PHA films was used to gauge how well they were degrading. The degradation of the PHA film above the surface of the soil nearly finished after being buried in the ground for eight weeks and degraded faster. In comparison the PHA film buried deep in soil degraded faster in less than 8 weeks as it was easier for microbes to attack on PHA buried deep in soil.
Based on studies, it was observed that PHA film buried in soil had more consistent degradation rate, observed from week 1 to week 7. This may be because deep in soil is level, which likely leads to a more uniform distribution of bacteria.
The PHA films' surface appearance amply demonstrated how quickly buried PHA film was degraded. It was clear that holes and edges were forming by week three. The degradation of PHA films occurred by surface erosion mechanisms, wherein microorganisms adhered to the porous portion of the film and then secreted depolymerase enzymes, which catalyzed the polymer’s breakdown. It is necessary to do additional research to ascertain whether the alterations in the soil microbial community brought about by PHA degradation are advantageous to the soil’s fertility.
|
Figure 1 Workflow diagram of current study. Diagram shows screening of PHA accumulating colonies to PHA isolation. |
|
Figure 2 FTIR spectrum showing an intense peak at 1722 cm-1 and some stretches in range of 1330-1200: (a) FTIR spectra of standard PHA (Sigma Aldrich); (b) FTIR spectra of PHA produced by BM2. |

|
Figure 3 Degradation of PHA in soil: (a) PHA film produced by BM2; (b) PHA film immersed in the dry soil; (c) PHA film degraded partially in wet soil after 15 days; (d) PHA film mostly degraded in wet soil after 30 days. |
|
Table 6 PHA Yield fro m NB at Different Co ncentratio ns o f Wasted Edible Oil Supplement |
The collected samples of food waste from Rawalpindi, Lahore, Gujranwala, Multan, Okara and Sahiwal and 42 samples showed pink/orange colonies in the Nile blue under UV irradiations. We have identified Bacillus megaterium, Bacillus cereus, Bacillus pumulisBacillus BA-126, Enterobacter, Citrobacter, Halomonas and Klebsiella from the different food waste materials. Several wasted food source like wasted rice, potato, fruit, cheese whey molasses and frying oil were used as supplement in NB and MSM. Molasses and waste frying oil has provided highest yield of 56.89% and 53.9% PHA to cell mass at 3 and 2% supplement in NB medium. The yield of PHA using 40% hydrolyzed wasted potato media and wasted rice media supplemented with 60% nutritional broth media were 0.6 g/100 ml and 0.86 g/100 mL (40% and 57.33%) respectively. FTIR analysis showed similar spectra and intense peaks at 1722 cm-1 and some stretches in the range of 1330-1200 cm-1 which is similar to PHA isolated from other Bacillus species.
- 1. Koller, M. Biodegradable and Biocompatible Polyhydroxy-alkanoates (PHA): Auspicious Microbial Macromolecules for Pharmaceutical and Therapeutic Applications. Molecules. 2018, 23.
-

- 2. Braunegg, G.; Bona, R.; Koller, M. Sustainable Polymer Production. Polym. Plastics Technol. Eng. 2004, 43, 1779-1793.
-

- 3. Zhang, Y.; Wusiman, A.; Liu, X.; Wan, C.; Lee, DJ.; Tay, J. Polyhydroxyalkanoates (PHA) Production from Phenol in An Acclimated Consortium: Batch Study and Impacts of Operational Conditions. J. Biotechnol. 2018, 267, 36-44.
-

- 4. Costa, S. S.; Miranda, A. L.; de Morais, M. G.; Costa, J. A. V.; Druzian, J. I. Microalgae as Source of Polyhydroxyalkanoates (PHAs) - A Review. Int. J. Biological Macromol. 2019, 131, 536-47.
-

- 5. Anjum, A.; Zuber, M.; Zia, K. M.; Noreen, A.; Anjum, M. N.; Tabasum, S. Microbial Production of Polyhydroxyalkanoates (PHAs) and Its Copolymers: A Review of Recent Advancements. Int. J. Biological Macromol. 2016, 89, 161-74.
-

- 6. Amaro, T.; Rosa, D.; Comi, G.; Iacumin, L. Prospects for the Use of Whey for Polyhydroxyalkanoate (PHA) Production. Front. Microbiol. 2019, 10, 992.
-

- 7. Arias, D. M.; Garcia, J.; Uggetti, E. Production of Polymers by Cyanobacteria Grown in Wastewater: Current Status, Challenges and Future Perspectives. New Biotechnol. 2020, 55, 46-57.
-

- 8. Kadouri, D.; Jurkevitch, E.; Okon, Y.; Castro-Sowinski, S. Ecological and Agricultural Significance of Bacterial Polyhydroxyalkanoates. Crit. Rev. Microbiol. 2005, 31, 55-67.
-

- 9. Wu, Q.; Wang, Y.; Chen, G. Q. Medical Application of Microbial Biopolyesters Polyhydroxyalkanoates. Artif. Cells Blood Substit. Immobil. Biotechnol. 2009, 37, 1-12.
-

- 10. Huang, Y. T.; Chen, P. L.; Semblante, G. U.; You, S. J. Detection of Polyhydroxyalkanoate-accumulating Bacteria from Domestic Wastewater Treatment Plant Using Highly Sensitive PCR Primers. J. Microbiol. Biotechnol. 2012, 22, 1141-1147.
-

- 11. Shrivastav, A.; Kim, H. Y.; Kim, Y. R. Advances in the Applications of Polyhydroxyalkanoate Nanoparticles for Novel Drug Delivery System. BioMed Res. Int. 2013, 2013, 581684.
-

- 12. Brigham, C. J.; Kurosawa, K.; Rha, C.; Sinskey, A. J. Bacterial Carbon Storage to Value Added Products. J. Microb. Biochem. Technol. 2011, 83, 1-13.
- 13. Budde, C. F.; Riedel, S. L.; Willis, L. B.; Rha, C.; Sinskey, A. J. Production of Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from Plant Oil by Engineered Ralstonia Eutropha Strains. Appl. Environ. Microbiol. 2011, 77, 2847-2854.
-

- 14. Singh, A. K.; Mallick, N. Advances in Cyanobacterial Polyhydr- oxyalkanoates Production. FEMS Microbiol. Lett. 2017, 364.
-

- 15. Lim, J.; You, M.; Li, J.; Li, Z. Emerging Bone Tissue Engineering via Polyhydroxyalkanoate (PHA)-based Scaffolds. Mater. Sci. Eng. C 2017, 79, 917-929.
-

- 16. Grigore, M. E.; Grigorescu, R. M.; Iancu, L.; Ion, R. M.; Zaharia, C.; Andrei, E. R. Methods of Synthesis, Properties and Biomedical Applications of Polyhydroxyalkanoates: A Review. J. Biomater. Sci. Polym. Ed. 2019, 30, 695-712.
-

- 17. Cammas, S.; Bear, M. M.; Moine, L.; Escalup, R.; Ponchel, G.; Kataoka, K.; Guérin, P. Polymers of Malic Acid and 3-alkylmalic Acid as Synthetic PHAs in the Design of Biocompatible Hydrolyzable Devices. Int. J. Biol. Macromol. 1999, 25, 273-282.
-

- 18. Niamsiri, N.; Delamarre, S. C.; Kim, Y. R.; Batt, C. A. Engineering of Chimeric Class II Polyhydroxyalkanoate Synthases. Appl. Environ. Microbiol. 2004, 70, 6789-6799.
-

- 19. Grage, K.; Jahns, A. C.; Parlane, N.; Palanisamy, R.; Rasiah, I. A.; Atwood, J. A.; Rehm, B. H. Bacterial Polyhydroxyalkanoate Granules: Biogenesis, Structure, and Potential Use as Nano-/micro-beads in Biotechnological and Biomedical Applications. Biomacromolecules. 2009, 10, 660-669.
-

- 20. Wei, X.; Hu, Y. J.; Xie, W. P.; Lin, R. L.; Chen, G. Q. Influence of poly(3-hydroxybutyrate-co-4-hydroxybutyrate-co-3-hydroxyhexanoate) on Growth and Osteogenic Differentiation of Human Bone Marrow-derived Mesenchymal Stem Cells. J. Biomed. Mater. Res. A 2009, 90, 894-905.
-

- 21. Ray, S.; Kalia, V. C. Biomedical Applications of Polyhydroxyalkanoates. Indian J. Microbiol. 2017, 57, 261-269.
-

- 22. Nomura, C. T.; Tanaka, T.; Gan, Z.; Kuwabara, K.; Abe, H.; Takase, K.; Taguchi, K.; Doi, Y. Effective Enhancement of Short-chain-length-medium-chain-length Polyhydroxyalkanoate Copolymer Production by Coexpression of Genetically Engineered 3-ketoacyl-acyl-carrier-Protein Synthase III (fabH) and Polyhydroxyalkanoate Synthesis Genes. Biomacromolecules 2004, 5, 1457-1464.
-

- 23. Matsumoto, K.; Murata, T.; Nagao, R.; Nomura, C. T.; Arai, S.; Arai, Y.; Takase, K.; Nakashita, H.; Taguchi, S.; Shimada, H. Production of Short-chain-length/medium-chain-length Polyhydroxyalkanoate (PHA) Copolymer in the Plastid of Arabidopsis Thaliana Using an Engineered 3-ketoacyl-acyl Carrier Protein Synthase III. Biomacromolecules 2009, 10, 686-690.
-

- 24. Zinn, M.; Witholt, B.; Egli, T. Occurrence, Synthesis and Medical Application of Bacterial Polyhydroxyalkanoate. Adv. Drug Deliv. Rev. 2001, 53, 5-21.
-

- 25. Chen, G. Q. A Microbial Polyhydroxyalkanoates (PHA) Based Bio- and Materials Industry. Chem. Soc. Rev. 2009, 38, 2434-2446.
-

- 26. Mannina, G.; Presti, D.; Montiel-Jarillo, G.; Carrera, J.; Suarez-Ojeda, M. E. Recovery of Polyhydroxyalkanoates (PHAs) from Wastewater: A Review. Bioresour. Technol. 2020, 297, 122478.
-

- 27. Yao, J.; Xiao, X. Y.; Wang, M.; Zhang, Q.; Chen, Y.; Gou, M.; Xia, Z. Y.; Tang, Y. Q. A Review of Low-cost Production of Polyhydroxyalkanoates: Strategies, Challenges, and Perspectives. Bioresour. Technol. 2025, 433, 132745.
-

- 28. Lau, N. S.; Sudesh, K. Revelation of the Ability of Burkholderia sp. USM (JCM 15050) PHA Synthase to Polymerize 4-hydroxybutyrate Monomer. AMB Express 2012, 2, 41.
-

- 29. Berlanga, M.; Minana-Galbis, D.; Domenech, O.; Guerrero, R. Enhanced Polyhydroxyalkanoates Accumulation by Halomonas spp. in Artificial Biofilms of Alginate Beads. Int. Microbiol. 2012, 15, 191-199.
-

- 30. Johnston, B.; Jiang, G.; Hill, D.; Adamus, G.; Kwiecien, I.; Zieba, M.; Sikorska, W.; Green, M.; Kowalczuk, M.; Radecka, I. The Molecular Level Characterization of Biodegradable Polymers Originated from Polyethylene Using Non-oxygenated Polyethylene Wax as a Carbon Source for Polyhydroxyalkanoate Production. Bioengineering 2017, 4, 3.
-

- 31. Biernacki, M.; Marzec, M.; Roick, T.; Patz, R.; Baronian, K.; Bode, R.; Kunze, G. Enhancement of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Accumulation in Arxula Adeninivorans by Stabilization of Production. Microb. Cell Fact. 2017, 16, 144.
-

- 32. Yang, C.; Zhang, W.; Liu, R.; Zhang, C.; Gong, T.; Li, Q.; Wang, S.; Song, C. Analysis of Polyhydroxyalkanoate (PHA) Synthase Gene and PHA-producing Bacteria in Activated Sludge that Produces PHA Containing 3-hydroxydodecanoate. FEMS Microbiol. Lett. 2013, 346, 56-64.
-

- 33. Srivastava, S. K.; Tripathi, A. D. Effect of Saturated and Unsaturated Fatty Acid Supplementation on Bio-plastic Production Under Submerged Fermentation. Biotech 2013, 3, 389-397.
-

- 34. Chen, G. Q.; Jiang, X. R. Engineering Bacteria for Enhanced Polyhydroxyalkanoates (PHA) Biosynthesis. Synth. Syst. Biotechnol. 2017, 2, 192-197.
-

- 35. Spiekermann, P.; Rehm, B. H.; Kalscheuer, R.; Baumeister, D.; Steinbuchel, A. A Sensitive, Viable-colony Staining Method Using Nile Red for Direct Screening of Bacteria That Accumulate Polyhydroxyalkanoic Acids and Other Lipid Storage Compounds. Arch. Microbiol. 1999, 171, 73-80.
-

- 36. Sambrook, J.; Russell, D. W. Molecular Cloning: A Laboratory Manual. 3rd ed.; Cold Spring Harbor Laboratory Press: New York, 2001.
- 37. Ramsay, J. A.; Berger, E.; Ramsay, B. A.; Chavarie, C. Recovery of Poly-3-hydroxyalkanoic Acid Granules by a Surfactant Hypochlorite Treatment. Biotechnol. Tech. 1990, 4, 221-226.
-

- 38. Jacquel, N.; Lo, C. W.; Wei, Y. H.; Wu, H. S.; Wang, S. S. Isolation and Purification of Bacterial Poly(3-hydroxyalkanoates). Biochem. Eng. J. 2008, 39, 15-27.
-

- 39. Tajima, K.; Igari, T.; Nishimura, D.; Nakamura, M.; Satoh, Y.; Munekata, M. Isolation and Characterization of Bacillus sp. INT005 Accumulating Polyhydroxyalkanoate (PHA) from Gas Field Soil. J. Biosci. Bioeng. 2003, 95, 77-81.
-

- 40. Liu, M.; Gonzalez, J. E.; Willis, L. B.; Walker, G. C. A Novel Screening Method for Isolating Exopolysaccharide-deficient Mutants. Appl. Environ. Microbiol. 1998, 64, 4600-4602.
-

- 41. Chang, Y. C.; Reddy, M. V.; Imura, K.; Onodera, R.; Kamada, N.; Sano, Y. Two-stage Polyhydroxyalkanoates (PHA) Production from Cheese Whey Using Acetobacter Pasteurianus C1 and Bacillus sp. CYR1. Bioengineering 2021, 8, 11.
-

- 42. Vu, D. H.; Akesson, D.; Taherzadeh, M. J.; Ferreira, J. A. Recycling Strategies for Polyhydroxyalkanoate-based Waste Materials: An Overview. Bioresour. Technol. 2020, 298, 122393.
-

- 43. Nielsen, C.; Rahman, A.; Rehman, A. U.; Walsh, M. K.; Miller, C. D. Food Waste Conversion to Microbial Polyhydroxyalkanoates. Microb. Biotechnol. 2017, 10, 1338-1352.
-

- 44. Tsang, Y. F.; Kumar, V.; Samadar, P.; Yang, Y.; Lee, J.; Ok, Y. S.; Song, H.; Kim, K. H.; Kwon, E. E.; Jeon, Y. J. Production of Bioplastic Through Food Waste Valorization. Environ. Int. 2019, 127, 625-644.
-

- 45. Zheng, Z.; Deng, Y.; Lin, X. S.; Zhang, L. X.; Chen, G. Q. Induced Production of Rabbit Articular Cartilage-derived Chondrocyte Collagen II on Polyhydroxyalkanoate Blends. J. Biomater. Sci. Polym. Ed. 2003, 14, 615-624.
-

- 46. Obruca, S.; Petrik, S.; Benesova, P.; Svoboda, Z.; Eremka, L.; Marova, I. Utilization of Oil Extracted from Spent Coffee Grounds for Sustainable Production of Polyhydroxyalkanoates. Appl. Microbiol. Biotechnol. 2014, 98, 5883-5890.
-

- 47. Pan, L.; Li, J.; Wang, R.; Wang, Y.; Lin, Q.; Li, C.; Wang, Y. Biosynthesis of Polyhydroxyalkanoate From Food Waste Oil by Pseudomonas Alcaligenes with Simultaneous Energy Recovery from Fermentation Wastewater. Waste Manag. 2021, 131, 268-276.
-

- 48. Xu, Z.; Pan, C.; Li, X.; Hao, N.; Zhang, T.; Gaffrey, M. J.; Pu, Y.; Cort, J. R.; Ragauskas, A. J.; Qian, W. J.; Yang, B. Enhancement of Polyhydroxyalkanoate Production by co-feeding Lignin Derivatives with Glycerol in Pseudomonas Putida KT2440. Biotechnol. Biofuels 2021, 14, 11.
-

- 49. Silva-Queiroz, S. R.; Silva, L. F.; Pradella, J. G.; Pereira, E. M.; Gomez, J. G. PHA (MCL) Biosynthesis Systems in Pseudomonas Aeruginosa and Pseudomonas Putida Strains Show Differences on Monomer Specificities. J. Biotechnol. 2009, 143, 111-118.
-

- 50. Khamkong, T.; Poomipuk, W.; Lumyong, S. Optimization of Production of Polyhydroxyalkanoates (PHAs) from Newly Isolated Ensifer sp. Strain HD34 by Response Surface Methodology. Processes 2022, 10, 1632.
-

- 51. Patil, T. D.; Ghosh, S.; Agarwal, A.; Patel, S. K. S.; Tripathi, A. D.; Mahato, D. K.; Kumar, P.; Slama, P.; Pavlik, A.; Haque, S. Author Correction: Production, Optimization, Scale up and Characterization of Polyhydoxyalkanoates Copolymers Utilizing Dairy Processing Waste. Sci. Rep. 2024, 14, 10919.
-

- 52. Gutschmann, B.; Maldonado Simoes, M.; Schiewe, T.; Schroter, E. S.; Munzberg, M.; Neubauer, P.; Bockisch, A.; Riedel, S. L. Continuous Feeding Strategy for Polyhydroxyalkanoate Production From Solid Waste Animal Fat at Laboratory- and Pilot-scale. Microb. Biotechnol. 2023, 16, 295-306.
-

- 53. Acedos, M. G.; Moreno-Cid, J.; Verdu, F.; Gonzalez, J. A.; Tena, S.; Lopez, J. C. Exploring the Potential of Slaughterhouse Waste Valorization: Development and Scale-up of a New Bioprocess for Medium-chain Length Polyhydroxyalkanoates Production. Chemosphere 2022, 287, 132401.
-

- 54. Van Thuoc, D.; My, D. N.; Loan, T. T.; Sudesh, K. Utilization of Waste Fish Oil and Glycerol as Carbon Sources for Polyhydroxyalkanoate Production by Salinivibrio sp. M318. Int. J. Biol. Macromol. 2019, 141, 885-892.
-

- 55. Morya, R.; Andrianantenaina, F. H.; Pandey, A. K.; Yoon, Y. H.; Kim, S. H. Polyhydroxyalkanoate Production from Rice Straw Hydrolysate: Insights Into Feast-famine Dynamics and Microbial Community Shifts. Chemosphere 2023, 341, 139967.
-

- 56. Ali, I.; Jamil, N. Enhanced Biosynthesis of Poly(3-hydroxybutyrate) from Potato Starch by Bacillus Cereus Strain 64-INS in a Laboratory-scale Fermenter. Prep. Biochem. Biotechnol. 2014, 44, 822-833.
-

- 57. Rysbek, A.; Ramankulov, Y.; Kurmanbayev, A.; Richert, A.; Abeldenov, S. Comparative Characterization and Identification of Poly-3-hydroxybutyrate Producing Bacteria with Subsequent Optimization of Polymer Yield. Polymers 2022, 14, 353.
-

- 58. Ong, S. Y.; Sudesh, K. Effects of Polyhydroxyalkanoate Degradation on Soil Microbial Community. Polym. Degrad. Stab. 2016, 131, 9-19.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2024 Impact Factor : 0.6
- Indexed in SCIE
 This Article
This Article
-
2026; 50(1): 60-68
Published online Jan 25, 2026
- 10.7317/pk.2026.50.1.60
- Received on Jun 11, 2025
- Revised on Sep 12, 2025
- Accepted on Sep 15, 2025
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
- Conflict of Interest
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Muhammad Asif Rasheed
-
Department of Biosciences, COMSATS University Islamabad, Sahiwal Campus, Sahiwal 57000, Pakistan
- E-mail: asif.rasheed@cuisahiwal.edu.pk
- ORCID:
0000-0002-9886-1591








 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.