- Triclosan-Incorporated Natural Rubber Latex Foam: A New Approach to Antibacterial Performance
Rubber Engineering Program, Department of Interdisciplinary Engineering, Faculty of Engineering, Prince of Songkla University, Hat Yai, Songkhla 90110 Thailand
*Department of Rubber Technology and Polymer Science, Faculty of Science and Technology, Prince of Songkla University, Pattani Campus, Pattani, 94000, Thailand- 트리클로산 첨가 천연고무 라텍스 폼의 항균 특성 개선 연구
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
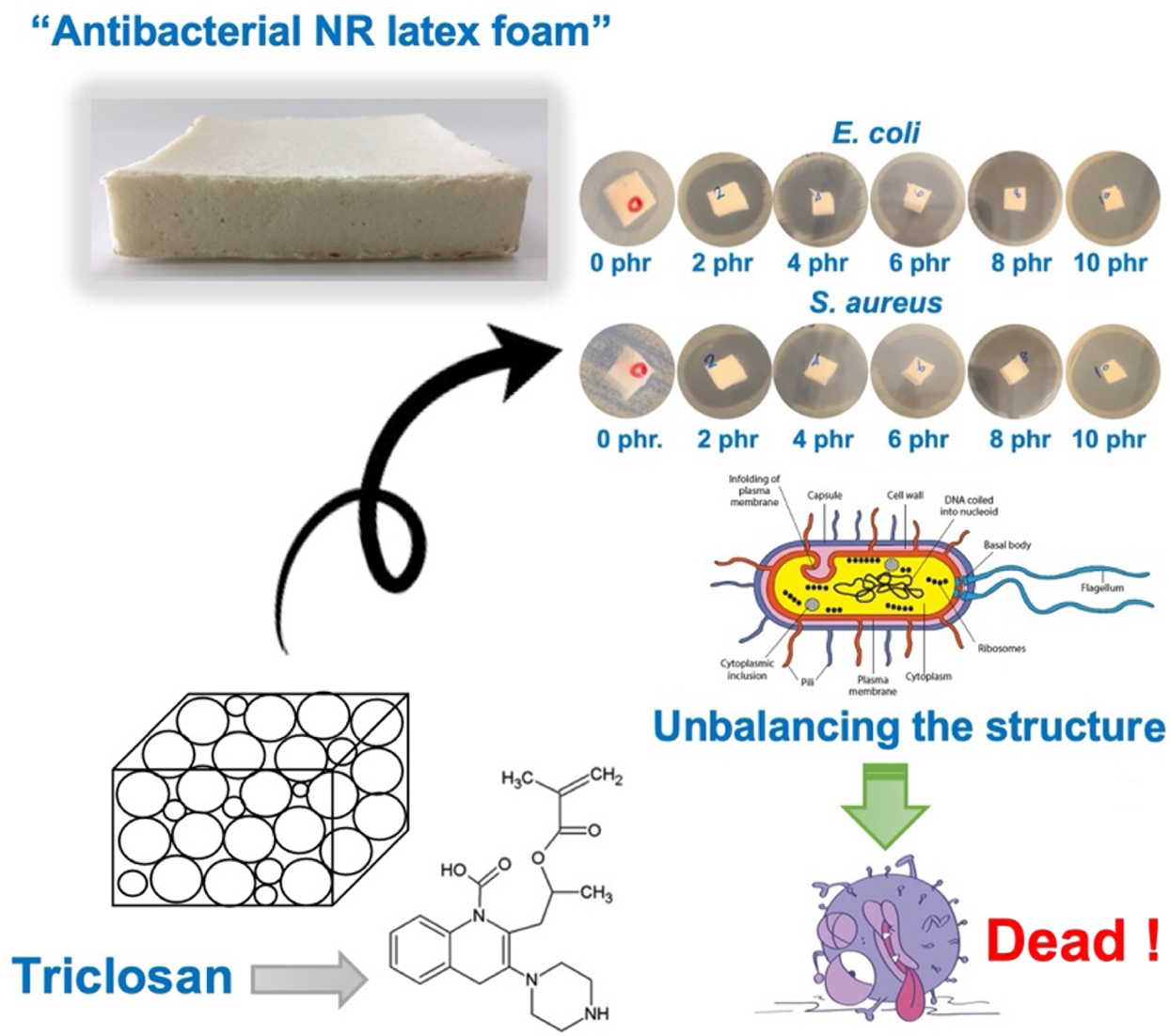
Natural rubber (NR) latex foam has been used in various products such as pillows, mattresses, and carpet underlays, to name a few. Its cellular structure may lead to the easy growth of micro-organisms. This may bring harmful bacteria to the users. Therefore, this work aimed to develop antibacterial NR latex foam and offered the basic formulation for preparing it. An antibacterial NR latex foam was prepared in the presence of Triclosan. The use of Triclosan affects antibacterial activities, where the clear zones of S. aureus and E. coli increased with the Triclosan content. A shorter contact time was achieved to kill the bacteria. The use of Triclosan at only 2 phr is sufficient to gain 100% killing efficiency in 8 h of contact time. This study offered resourceful information in fabricating an antibacterial NR latex foam incorporating Triclosan.
This work aimed to develop antibacterial NR latex foam in the presence of Triclosan. A minimum of 2 phr still provides complete killing efficiency after 8 h, making it a suitable and efficient choice for long-lasting antibacterial protection.
Keywords: natural rubber, antibacterial performance, triclosan, foam, cellular structure.
The authors would like to thank the Natural Rubber Innovation Research Institute, Prince of Songkla University, for the Invention Development Fund through grant No. SAT6201085S.
The authors declare that there is no conflict of interest.
Natural rubber (NR) latex is an interesting biopolymer derived from the Hevea brasiliensis tree, commonly known as the rubber tree. In today’s modern world, this naturally sourced material is widely used to manufacture various products that enhance convenience and comfort in daily life.1-3 NR latex foam is one of the NR latex applications that can be converted into various products such as pillows, mattresses, and carpets.4 These products are mostly in contact with the users while using them. This has brought consumers to increase their health consciousness of the hygienic conditions.5,6 Therefore, the demand for NR latex foam with antimicrobial properties has been steadily rising as consumers become increasingly aware of bacteria and their potential health risks. This trend has been particularly pronounced since the COVID-19 pandemic, prompting manufacturers to address consumer needs by introducing antimicrobial solutions across a wide range of applications.
The porous NR latex foam is one of the materials that is easier for bacteria to grow compared to non-porous materials, which is a significant reason why porous materials become a source of microbes after some period of use. Microbial contamination in the environment can impact human health, especially when they are pathogenic. So, preparing NR latex foam with antibacterial properties is an interesting area of research. Generally, there is a way to control bacterial contamination on surfaces of materials using chemical disinfectants from the groups of alcohols, glutaraldehyde, chlorine-containing compounds, and phenols.7,8 These chemicals are commonly used for direct cleaning rather than being incorporated into polymers. The possible route is to find non-toxic chemicals to inhibit microorganisms and incorporating them into polymer materials.
Various antibacterial agents have been used in NR and other polymers, including silver (Ag) nanoparticles,9,10 chitosan,11,12 essential oils,13 and to name a few. Each has notable strengths and drawbacks. Ag nanoparticles exhibit strong antimicrobial activity but suffer from high cost, cytotoxicity concerns, and agglomeration issues that hinder uniform performance. Chitosan is biodegradable and biocompatible; however, its hydrophilicity reduces compatibility with NR and limits its long-term effectiveness. Essential oils are sustainable and broadly antimicrobial, but their volatility, thermal instability, and odor restrict practical use in NR processing. The primary considerations for using biocides in materials are high compatibility, long-term stability, and tolerance under processing conditions.
Triclosan is one among several choices. Triclosan is a single-hydroxyl group trichlorinated diphenyl ether antifungicides.14-16 Comparing the overall aspects, triclosan offers a unique balance of advantages: it is chemically stable, broadly effective against bacteria, easily dispersed within the NR matrix, cost-efficient compared to metallic agents, and retains its activity under processing conditions without compromising rubber properties. These attributes make triclosan a particularly attractive antibacterial agent for NR requiring durable and reliable protection. Triclosan is a widely used antimicrobial agent. Prapruddivongs and Sombatsompop17 found that the use of Triclosan in polylactic acid (PLA) resulted in an antimicrobial activity against E. coli, with inhibition rates of up to 83.4%. Zhang et al.18 modified the surface of polyvinyl chloride (PVC) with plasma treatment before coating it with Triclosan. This method also enhanced the antibacterial effectiveness of the PVC. Similarly, Asadinezhad et al.19 used Triclosan in PVC and chemically modified the polymer surface. The resulting product showed significant antibacterial efficacy.
Therefore, this study also outlines a potential formulation strategy to minimize the effect of Triclosan on the physical properties of NR latex foam. The findings will serve as a foundation for further development of antibacterial foam. Overall, this work represents a promising step toward the fabrication of antibacterial NR latex foam incorporating triclosan.
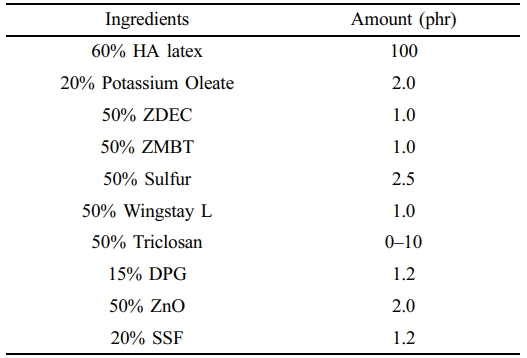
Materials. High Ammonia (HA) centrifuged latex was supplied by Yala Latex Industry Co., Ltd., based in Yala, Thailand. Triclosan was obtained from Chemipan Corporation Co., Ltd., Bangkok, Thailand. Additional ingredients including 50% Wingstay L, 50% zinc 2-mercaptobenzothiazole (ZMBT), 50% zinc diethyl dithiocarbamate (ZDEC), and 20% potassium oleate, 15% diphenylguanidine (DPG), 50% sulphur, and 50% sodium silicofluoride (SSF), were sourced from Siamnavakam Co., Ltd., located in Samut Prakan, Thailand.
Preparation of Antibacterial NR Latex Foam. Table 1 shows the compounding formulation for making antibacterial NR latex foam. The content of each ingredient is represented in the unit of part per hundred rubber or phr. All the ingredients mixed with centrifuged HA latex are prepared in dispersion and emulsion forms. The foam processing is referred to as the Dunlop process, where the SSF was used as the primary gelling agent. First, HA was added to the cake beater and stirred for 3 min to evaporate it. Next, 20% potassium oleate, 50% ZDEC, 50% ZMBT, 50% Wingstay L, and 50% sulfur were slowly added. The speed was then increased until reaching the required volume, which was approximately 5 min. Afterward, the 50% triclosan was added depending on their respective amounts. The mixing time depended on the amount of Triclosan, ranging from 2–4 min. Next, the 50% DPG and 50% ZnO, and 20% SSF were subsequently added, and the mixture was beaten for an additional 1 min. After that, the ungelled foam was promptly transferred into an aluminium mould and left to gel for less than 2 min at room temperature. The gelled foam was steam-cured for 45 min at 100 ℃. Finally, the foam was taken out of the mould and given a thorough water wash to get rid of any remaining soap and unreacted materials. The cleaned NR latex foam was then dried for 24 h at 60 ℃ in a hot air oven.
Measurement of Physical Properties. The density of the foam was determined based on weight per unit volume. The foam samples were cut into a cubic shape with dimensions of 3.0 × 3.0 × 3.0 cm3, weighed, and their volume calculated accordingly.
The cellular structure of the samples was screened using a light microscope at 40× magnification. The image was then captured and imported to the ImageJ software to measure the cell size.
The compressive strength of the NR latex foam was measured following ASTM D575. The test was conducted by compressing a specimen with dimensions of 10.0 × 10.0 × 2.5 cm3 using a universal testing machine (Tinius Olsen, H10KS) until the thickness was reduced by 25%. The result was reported in terms of the compressive stress (force per unit area) at 25% deformation.
The compression set was measured according to ASTM D395. A specimen with dimensions of 5.0 × 5.0 × 2.5 cm3 was compressed to 50% of its original thickness at 100 ℃. After 70 h, the load was removed, and the sample thickness was measured after allowing it to recover for 30 min. The compression set was then calculated using the standard formula as follows;
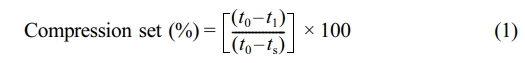
where t0 is the original thickness, t1 is the recovered thickness, and ts is the thickness of the spacer.
The raw outputs from compression set and compressive strength were statistically analyzed with the SigmaPlot program (SPSS Inc., IL, USA). Differences among groups were examined by using a one-way analysis of variance followed by a Tukey comparisons test. Differences were considered to be statistically significant at p-values less than 0.05.
Antibacterial Study. Both qualitative and quantitative antibacterial activities were tested in this study. All antibacterial assays were performed in triplicate to ensure reproducibility and reliability of the results. For the first method, the samples were placed at separate positions on Mueller-Hinton Agar (MHA) plates under hygienic conditions. A second thin layer of MHA (10 mL) was applied on the sample. A 15 mL of MHA was also added to the sterilized Petri dishes for the bottom layer. The optical density (OD) at 600 nm was used to measure the bacterial concentration, where an OD of 0.3 indicated a bacterial concentration of 1×108 CFU/mL. Then, 100 µL of solutions containing Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli) were evenly distributed on the MHA plate surface. The inoculated plates were incubated at 37 ℃ for 24 h to observe the zone of inhibition.
To conduct quantitative analysis, the test culture was first grown on nutritional agar (NA) medium and then incubated for 18 to 24 h at 37 ℃. Each colony was then transferred to Mueller-Hinton Broth (MHB) and incubated at 37 ± 0.5 ℃ for 3 h. The bacterial suspension's turbidity was adjusted to meet the 0.5 McFarland requirement. Then, 9 mL of MHB medium containing the test samples was mixed with 100 µL of the produced bacterial suspension. The suspension was incubated for 0, 2, 4, 6, 8, and 24 h at 37 ± 0.5 ℃. Upon incubation, the samples were plated onto NA medium, serially diluted ten times with 0.85% normal saline, and incubated for an entire night at 37 ± 0.5 ℃. Counting colony-forming units (CFU/mL) allowed us to determine the quantity of surviving bacteria. Parallel control experiments were carried out, and the outcomes were compared. The percent reduction of bacterial count was based on the following equation.
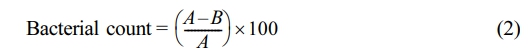
where A is the average bacterial count after a certain contact time, and B is the average bacterial count before the test. The negative value indicates the reduction of bacteria.
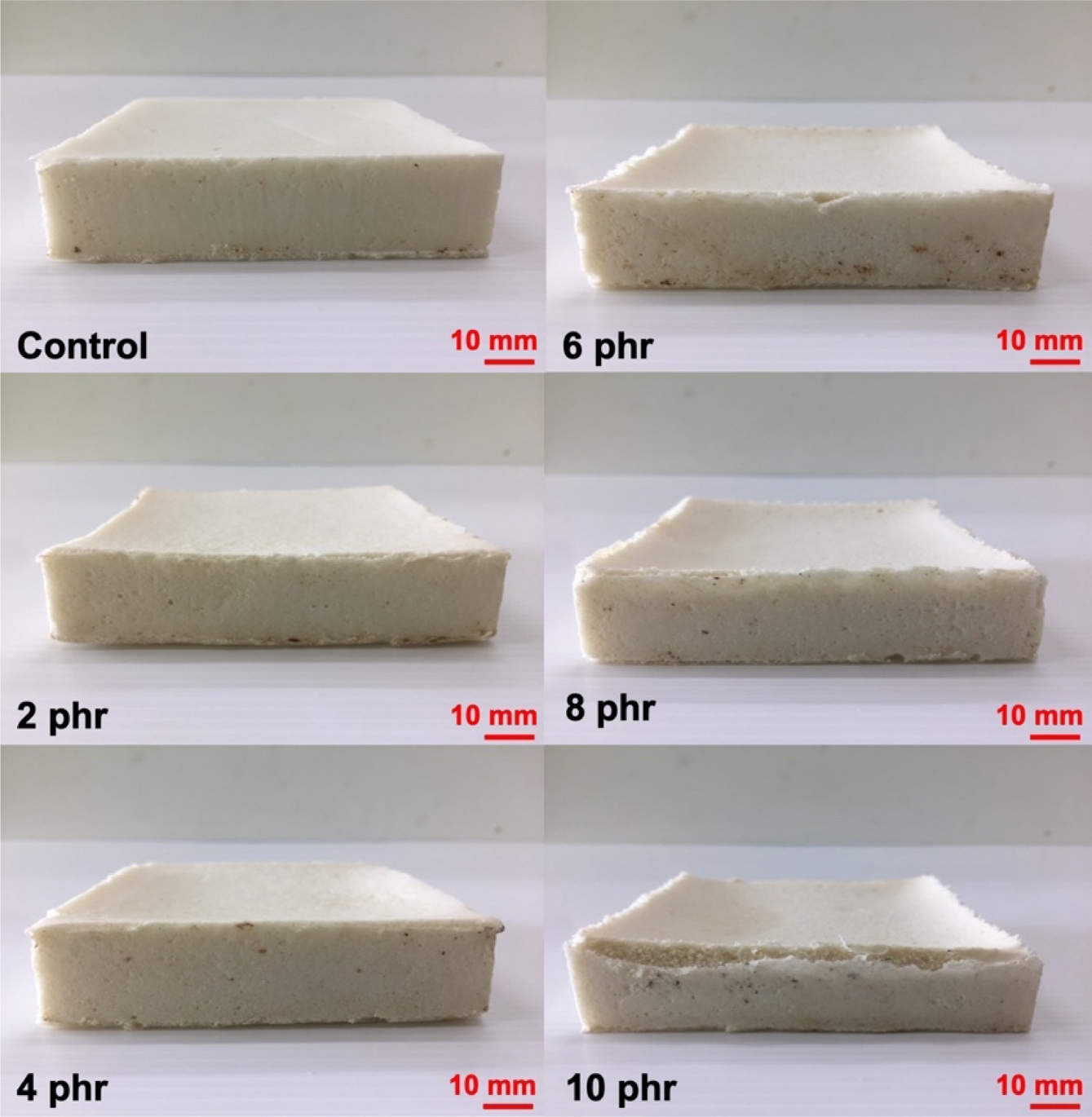
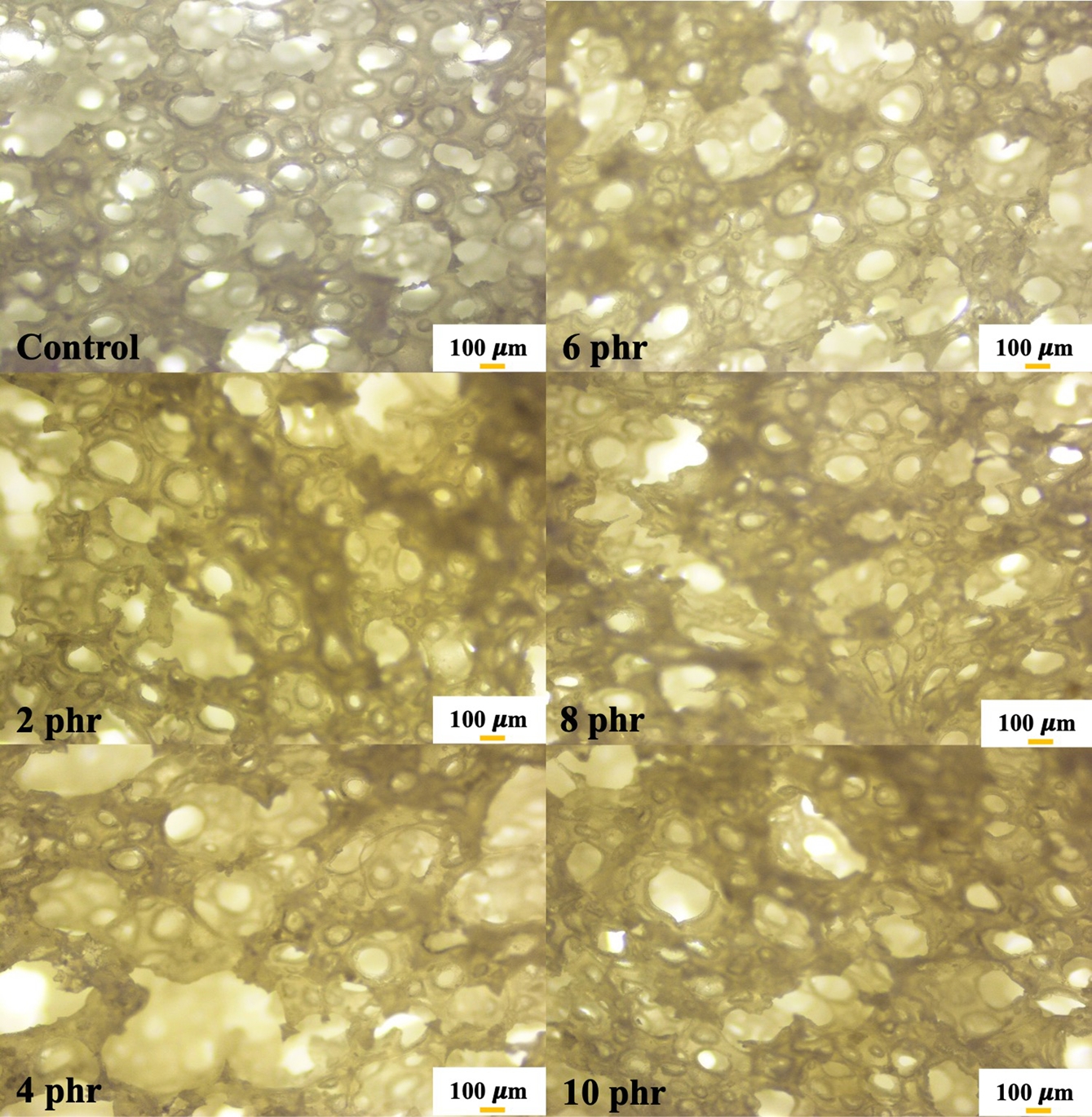
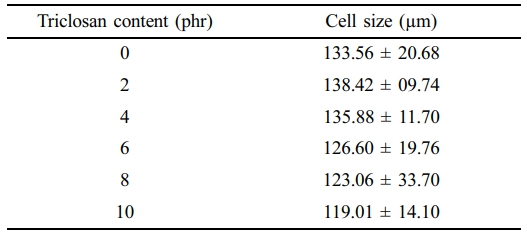
Physical Appearance and Morphology. Figure 1 presents the physical appearance of NR foams containing triclosan as an antibacterial agent. The physical appearance shows a noticeable collapse of the sponge structure corresponding to the triclosan content. The foam structure becomes less refined, likely due to the addition of triclosan, a solid-phase compound, into the latex. This may have disrupted the cell walls, making them more prone to breakage and resulting in structural collapse after vulcanization. Figure 2 shows microscopic images at 400× magnification that further reveal a reduction in cell size as the triclosan content increases. This is attributed to foam breakage and collapse, as previously mentioned. The cell sizes of NR latex foams were also measured and reported in Table 2.
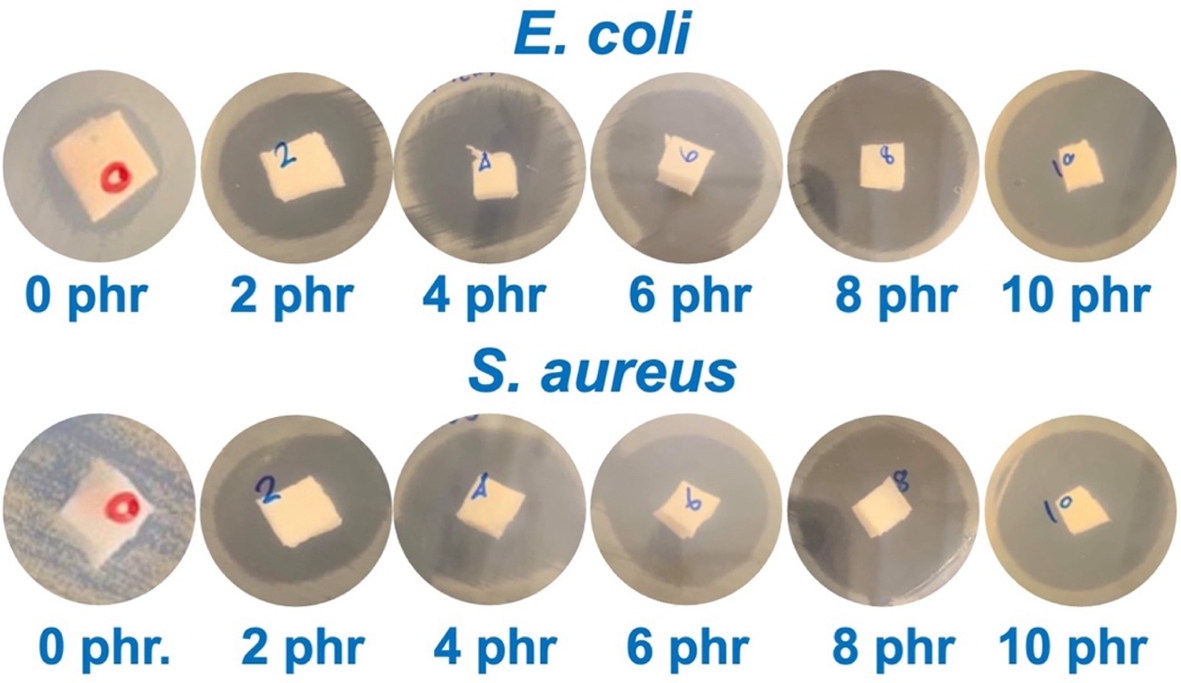
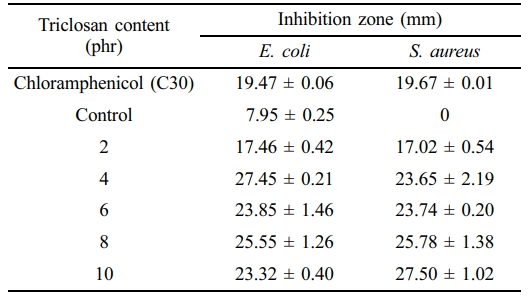
Antibacterial Performance. Table 3 shows the effect of triclosan content on the inhibition zone against S. aureus and E. coli bacteria in NR. The photographs during the test and the inhibition zone are shown in Figure 3. The experiment found that the inhibition zone increased with the concentration of triclosan, indicating the antibacterial effect of triclosan, which can inhibit both bacterial strains. There is extensive research on the use of the antibacterial agent triclosan due to its high antibacterial efficacy. Triclosan is incorporated into various chemical products such as toothpaste, mouthwash, toilet cleaners, etc. In addition, triclosan has been used in various polymers to study its antibacterial effectiveness. For example, Silapasorn et al.20 studied the use of triclosan in different plastics and found that it provided improved antibacterial properties with increasing triclosan concentration. Prapruddivongs et al.21 studied the use of triclosan as an antibacterial agent for polylactic acid and found it to be equally effective. However, the mechanism of antibacterial action of triclosan in polymer materials has not yet been reported in research. On the other hand, in the medical field, the antibacterial mechanism of triclosan has been widely reported.
In terms of the release mechanism, triclosan is physically blended into latex foam. It means triclosan is present within the latex matrix as a free additive, not covalently immobilized. Latex foams are porous structures with large surface areas, which can facilitate the migration of small molecules. This provides an initial antimicrobial surface layer. Petersen15 further reported that triclosan, when added to polymers, exhibits extremely low release into aqueous solutions due to its non-polar nature and the compatibilization within the polymer matrix. This results in a more uniform stability of triclosan in the solid state, allowing it to function effectively as an antimicrobial additive while minimizing solubility.
Bhargara and Leonard22 reported the antibacterial mechanism of triclosan, stating that when triclosan penetrates the bacterial cell wall, it inhibits protein synthesis in the bacteria. McMurry et al.23 reported that triclosan directly disrupts the fatty acid synthesis process in bacteria, which is a key component of phospholipids within the cell membrane structure and plays an important role in various metabolic processes within cells and tissues. This inhibition of fatty acid synthesis was confirmed by Larson et al.24 The chemical structure of triclosan can react directly with Enoyl-acyl, an important enzyme in fatty acid synthesis that serves as an energy source for bacteria, leading to difficulty in fatty acid synthesis. As a result, the bacterial inhibition zone increases with the concentration of triclosan.
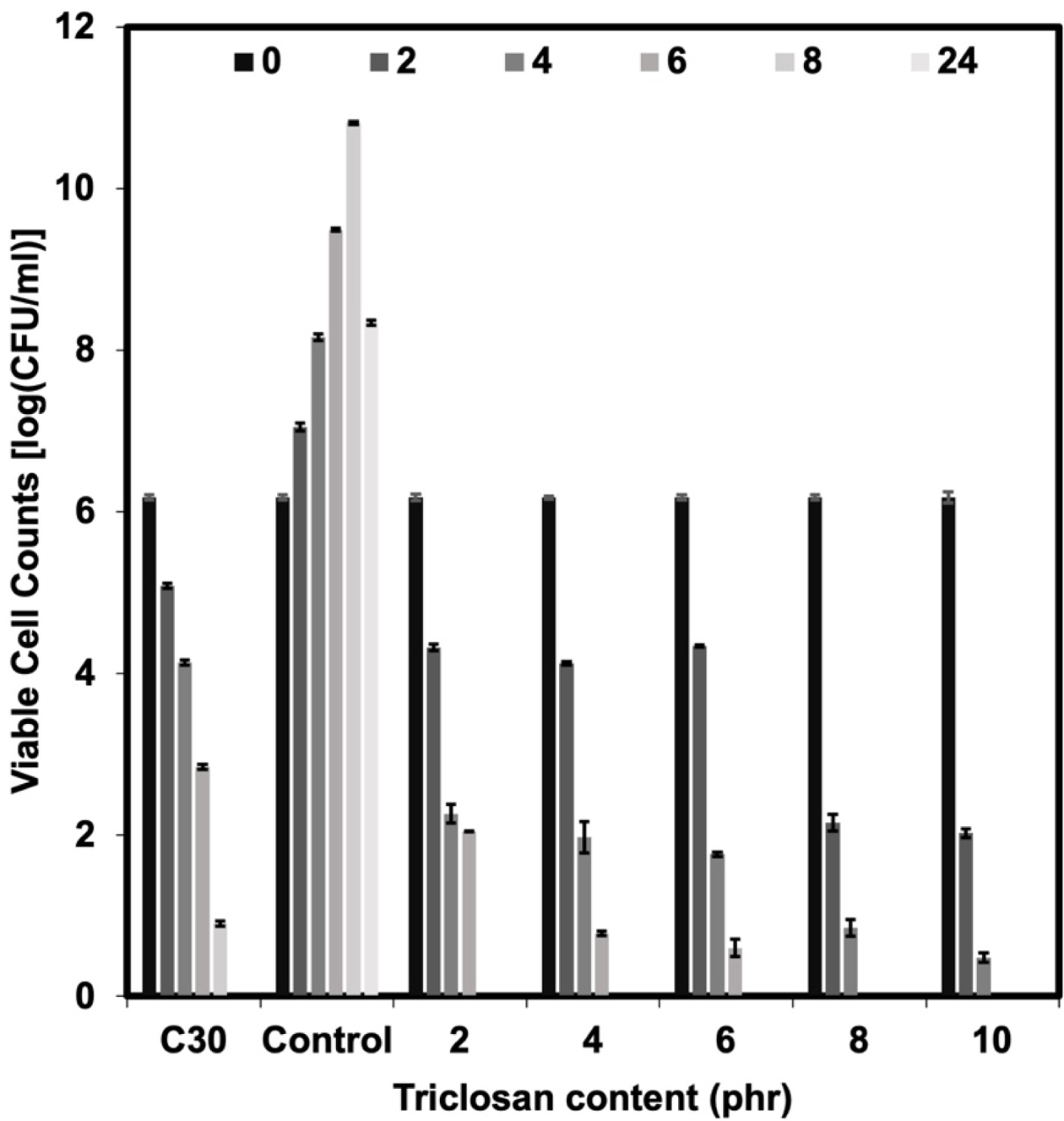
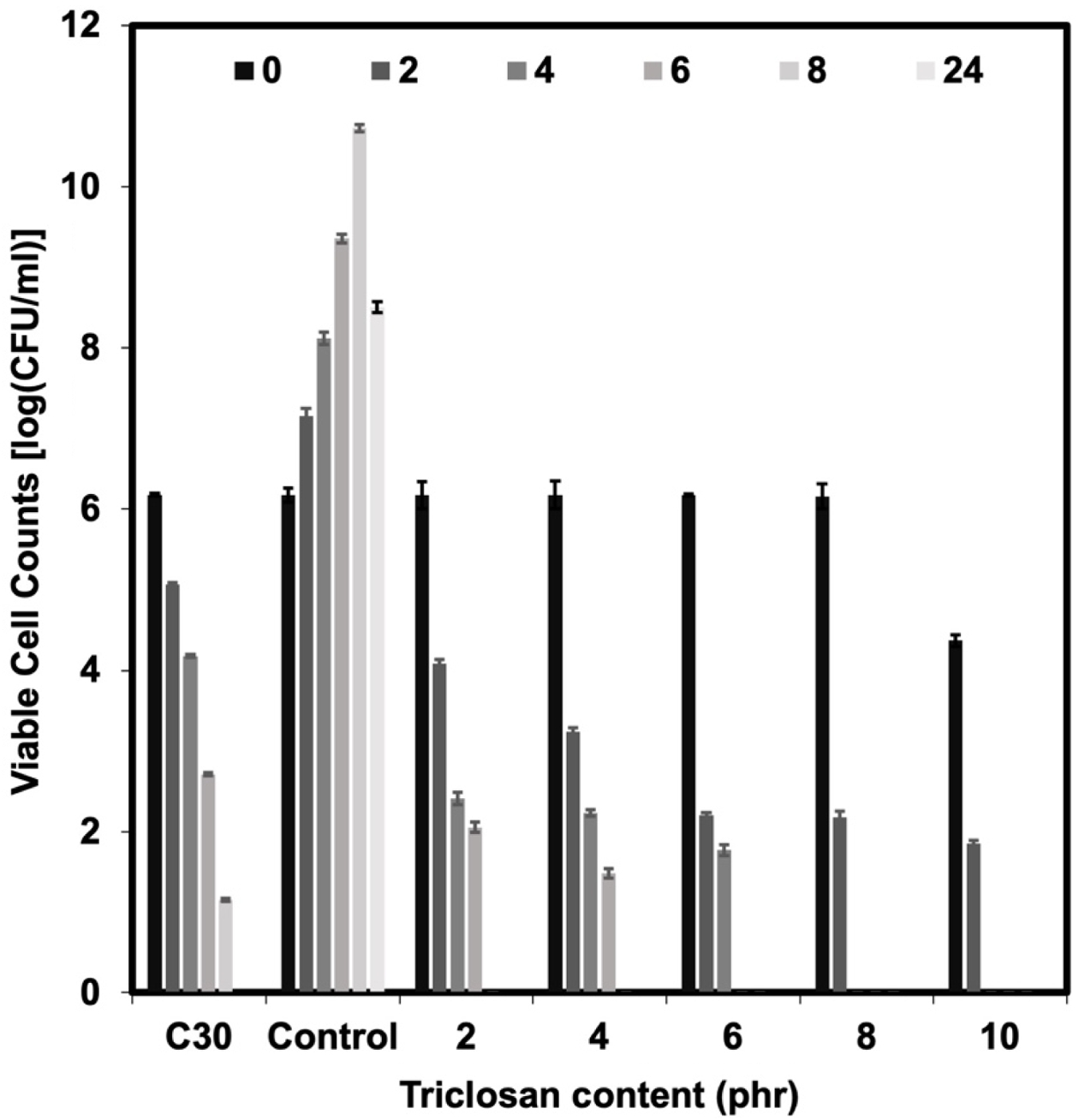
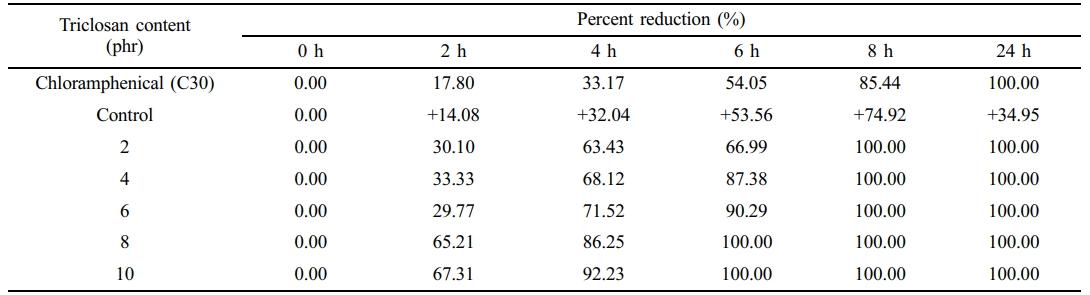
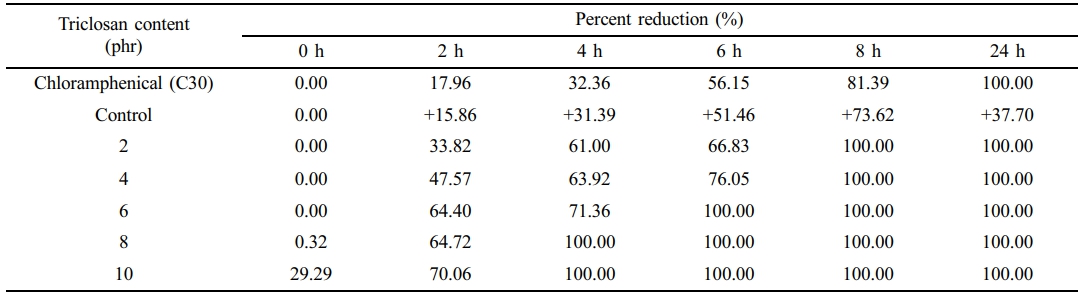
The bacterial survival count is consistent with the bacterial inhibition zone test (see Figures 4 and 5). The experiment found that no bacteria were detected surviving after 8 h onwards for the NR latex foam containing 2 phr triclosan. However, when considering the effects of contact times, it was found that after 4 h of contact time, no surviving bacteria could be detected in the NR latex foams containing 8–10 phr triclosan. This is consistent with the previous experimental results, where both bacterial strains showed similar trends. Based on the quantitative results, two key insights can be drawn from this experiment, depending on the design criteria. Using triclosan at 8 phr is recommended due to its faster killing efficiency. However, a minimum concentration of 2 phr is also effective, achieving 100% bacterial reduction with at least 8 h of contact time.
Tables 4 and 5 show the calculation of the percent reduction of E. coli and S. aureus against Chloramphenicol and the NR latex foam samples prepared at various Triclosan contents. The positive (+) symbol shows an increase in bacterial count. It was found that increasing the content of triclosan resulted in a lower percent survival of bacteria. It can be observed that triclosan inhibited the growth of the Gram-positive S. aureus more effectively than the Gram-negative E. coli. E. coli was 100% reduced at a triclosan content of 8 phr after 6 h of contact time, while S. aureus was 100% reduced at only 4 h of contact time when a similar content of triclosan was used. This demonstrates the antibacterial ability of triclosan, particularly against the Gram-positive S. aureus, through the antibacterial mechanism mentioned earlier.
Physical Properties. Table 6 shows the effect of triclosan content on the foam density, compression set, and compressive stress at 25% deformation. It was found that increasing the triclosan content slightly increased the foam density. This is because the addition of solid material into the NR latex foam increases the weight of the resulting foam, while the volume of the foam remains the same. The density of NR latex foam with triclosan content ranging from 2–10 phr was between 0.15–0.17 g/cm³.
The effect of triclosan content on the compression set was also studied. The compression set defines the foam's ability to recover after being compressed for a certain period. It was found to increase with the addition of triclosan. This is because triclosan acts as a filler, so increasing the triclosan content reduces the foam’s elasticity. This is consistent with the compressive stress at 25% deformation, which also showed a slight increase, indicating an increased ability of the foam to withstand force due to the addition of triclosan, a solid phase, into the NR latex foam. The experimental results are consistent with the report of Prapruddivongs et al.,25 who discussed the addition of triclosan at concentrations of 0–15 wt% in PLA. The experiment found that triclosan had a slight effect on improving the modulus of the polymer composites.
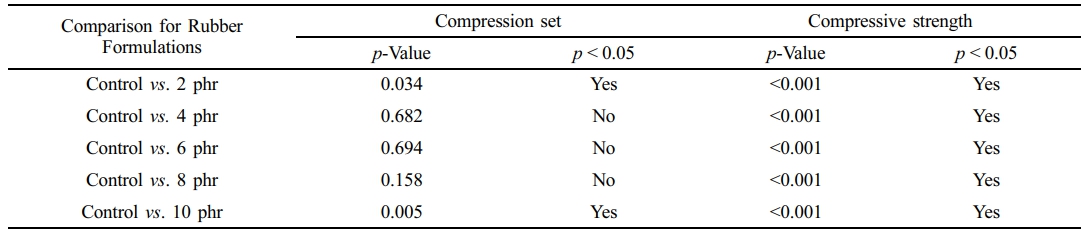
Table 7 presents the raw outputs from the statistical analysis. The results indicate a statistically significant difference in compression set between the control and NR foams containing 2 and 10 phr of triclosan, while the additions of 4, 6, and 8 phr did not differ significantly from the control. In contrast, for compressive strength, all treated groups (2, 4, 6, 8, and 10 phr) showed significantly higher values compared to the control. Although triclosan affected both properties, these variations are unlikely to fully reflect the performance of finished foamed products such as pillows and mattresses.
|
Figure 1 Physical appearance of the NR latex foam samples prepared at various Triclosan content. |
|
Figure 2 Optical images at 40× magnification NR latex foam samples prepared at various Triclosan content. |
|
Figure 3 Photos captured after antibacterial test against E. coli and S. aureus of the NR latex foam samples prepared at various triclosan content. |
|
Figure 4 Viable cell counts against E. coli of Chloramphenicol and the NR latex foam samples prepared at various triclosan content. |
|
Figure 5 Viable cell counts against S. aureus of Chloramphenicol and the NR latex foam samples prepared at various triclosan content. |
|
Table 2 Cell Size Measured From Optical Images of the NR Latex Foam Samples Prepared at Various Triclosan Content |
Remark: All pairwise comparisons show p > 0.05. |
|
Table 3 Inhibition Zone Measured After Antibacterial Test Against E. coli and S. aureus of the NR Latex Foam Samples Prepared at Various Triclosan Content |
|
Table 4 Percent Reduction of E. coli Against Chloramphenicol and the NR Latex Foam Samples Prepared at Various Triclosan Content |
Remark: + symbol shows an increase in bacterial count. |
|
Table 5 Percent Reduction of S. aureus Against Chloramphenicol and the NR Latex Foam Samples Prepared at Various Triclosan Content |
Remark: + symbol shows an increase in bacterial count. |
|
Table 6 Physical Characteristics of the NR Latex Foam Samples Prepared at Various Triclosan Content |
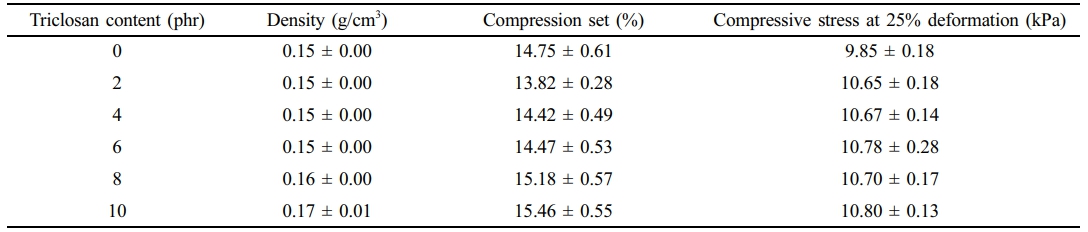
The idea of this study was to prepare the antibacterial NR latex foam. The antibacterial agent used in this work was Triclosan. The bacteria used in the test were divided into Gram-positive bacteria, Staphylococcus aureus (S. aureus), and Gram-negative bacteria, Escherichia coli (E. coli). Increasing the triclosan content resulted in smaller cell sizes of the foam but had little effect on the foam density, compression set, and compressive strength. The maximum inhibition zone for E. coli and S. aureus was 27.45 mm and 27.50 mm, respectively. Based on the quantitative results, two key insights can be drawn from this experiment, depending on the design criteria. The experiment revealed that triclosan at 8 phr provides rapid bactericidal action, while a lower concentration of 2 phr is sufficient to achieve 100% bacterial reduction after 8 h of contact. This lower concentration is strongly recommended to ensure sufficient antibacterial performance while minimizing additive content.
- 1. Karimi-Avargani, M.; Biria, D.; Dehghanifar, S.; Bazooyar, F.; Skrifvars, M. Accelerating Degradation of Natural Rubber Latex Gloves by A Consortium of Microorganisms in an Agricultural Soil Sample. Int. J. Environ. Sci. Technol. 2025, 22, 2601-2612.
-

- 2. Yip, E.; Cacioli, P. The Manufacture of Gloves from Natural Rubber Latex. J. Allergy Clin. Immunol. 2002, 110, S3-S14.
-

- 3. Guerra, N. B.; Pegorin, G. S. A.; Boratto, M. H.; de Barros, N. R.; de Oliveira Graeff, C. F.; Herculano, R. D. Biomedical Applications of Natural Rubber Latex From the Rubber Tree Hevea Brasiliensis. Mater. Sci. Eng. C 2021, 126, 112126.
-

- 4. Ramli, R.; Chai, A. B.; Ho, J. H.; Kamaruddin, S.; Rasdi, F. R. M.; De Focatiis, D. S. Specialty Natural Rubber Latex Foam: Foamability Study and Fabrication Process. Rubber Chem. Technol. 2022, 95, 492-513.
-

- 5. Zhang, N.; Cao, H. Enhancement of the Antibacterial Activity of Natural Rubber Latex Foam by Blending it with Chitin. Materials 2020, 13, 1039.
-

- 6. Mam, K.; Dangtungee, R. Effects of Silver Nanoparticles on Physical and Antibacterial Properties of Natural Rubber Latex Foam. Mater. Today: Proc. 2019, 17, 1914-1920.
-

- 7. Rutala, W.A.; Weber, D.J. Selection of the Ideal Disinfectant. Infect. Control Hosp. Epidemiol. 2014, 35, 855-865.
-

- 8. Tong, C.; Hu, H.; Chen, G.; Li, Z.; Li, A.; Zhang, J. Disinfectant Resistance in Bacteria: Mechanisms, Spread, and Resolution Strategies. Environ. Res. 2021, 195, 110897.
-

- 9. Masa, A.; Jehsoh, N.; Saiwari, S.; Dueramae, S.; Hayeemasae, N. Microwave-assisted Silver-doped Zinc Oxide Towards Antibacterial and Mechanical Performances of Natural Rubber Latex Film. Mater. Today Commun. 2023, 34, 105475.
-

- 10. Kwon, H. J.; Cha, J. R.; Gong, M. S. Facile Preparation of Antibacterial Plastisol/Ag Composites Based on Silver Carbamate and Their Properties. Polym. Korea. 2017, 41, 860.
-

- 11. Suteewong, T.; Wongpreecha, J.; Polpanich, D.; Jangpatarapongsa, K.; Kaewsaneha, C.; Tangboriboonrat, P. PMMA Particles Coated with Chitosan-silver Nanoparticles as a Dual Antibacterial Modifier for Natural Rubber Latex Films. Colloids Surf., B 2019, 174, 544-552.
-

- 12. Özakar, R. S.; Bingöl, M. S.; Adıgüzel, M. C.; Özakar, E. Preparation, Characterization of Chitosan-coated/uncoated Boron Nanoparticles and In Vitro Evaluation of Their Antimicrobial Effects. Polym. Korea. 2022, 46, 709-721.
-

- 13. Shamene, B.; Tee, S. M.; Rosli, N. A. Strategic Addition of Rosemary Essential Oil in Natural Rubber-toughened Poly(lactic acid)/Cinnamon Oil Blends for Antimicrobial Packaging. Future Foods 2024, 10, 100506.
-

- 14. Petersen, R. C. Computational Conformational Antimicrobial Analysis Developing Mechanomolecular Theory for Polymer Biomaterials in Materials Science and Engineering. Int. J. Comput. Mater. Sci. Eng. 2014, 3, 48.
-

- 15. Petersen, R. C. Triclosan Antimicrobial Polymers. AIMS Mol. Sci. 2016, 3, 88-103.
-

- 16. Dann, A. B.; Hontela, A. Triclosan: Environmental Exposure, Toxicity and Mechanisms of Action. Appl. Toxicol. 2011, 31, 285-311.
-

- 17. Prapruddivongs, C.; Sombatsompop, N. Roles and Evidence of Wood Flour as an Antibacterial Promoter for Triclosan-filled Poly (Lactic Acid). Compos. B: Eng. 2012, 43, 2730-2737.
-

- 18. Zhang, W.; Chu, P. K.; Ji, J.; Zhang, Y.; Liu, X.; Fu, R. K.; Ha, P. C.; Yan, Q. Plasma Surface Modification of Poly Vinyl Chloride for Improvement of Antibacterial Properties. Biomaterials 2006, 27, 44-51.
-

- 19. Asadinezhad, A.; Lehocký, M.; Sáha, P.; Mozetič, M. Recent Progress in Surface Modification of Polyvinyl Chloride. Materials 2012, 5, 2937-2959.
-

- 20. Silapasorn, K.; Sombatsompop, K.; Kositchaiyong, A.; Wimolmala, E.; Markpin, T.; Sombatsompop, N. Effect of Chemical Structure of Thermoplastics on Antibacterial Activity and Physical Diffusion of Triclosan Doped in Vinyl Thermoplastics and Their Composites with CaCO3. J. Appl. Polym. Sci. 2011, 121, 253-261.
-

- 21. Prapruddivongs, C.; Sombatsompop, N.; Jayaraman, K. Effect of Organoclay Incorporation on Mechanical, Barrier and Thermal Properties and Anti-bacterial Performance of PLA and PLA Composites with Triclosan and Wood Flour. Polym. Polym. Compos. 2014, 22, 643-652.
-

- 22. Bhargava, H. N.; Leonard, P. A. Triclosan: Applications and Safety. Am. J. Infect. Control. 1996, 24, 209-218.
-

- 23. McMurry, L. M.; Oethinger, M.; Levy, S. B. Triclosan Targets Lipid Synthesis. Nature 1998, 394, 531-532.
-

- 24. Larson, E. Guideline for Use of Topical Antimicrobial Agents. Am. J. Infect. Control. 1988, 16, 253-266.
-

- 25. Prapruddivongs, C.; Sombatsompop, N. Wood, Silver-substituted Zeolite and Triclosan as Biodegradation Controllers and Antibacterial Agents for Poly(Lactic Acid)(PLA) and PLA Composites. J. Thermoplast. Compos. Mater. 2017, 30, 583-598.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2024 Impact Factor : 0.6
- Indexed in SCIE
 This Article
This Article
-
2026; 50(1): 76-83
Published online Jan 25, 2026
- 10.7317/pk.2026.50.1.76
- Received on Jun 16, 2025
- Revised on Sep 1, 2025
- Accepted on Sep 4, 2025
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
- Conflict of Interest
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Nabil Hayeemasae
-
Department of Rubber Technology and Polymer Science, Faculty of Science and Technology, Prince of Songkla University, Pattani Campus, Pattani, 94000, Thailand
- E-mail: nabil.h@psu.ac.th
- ORCID:
0000-0002-9924-582X









 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.