- Facile Fabrication of Collector-Free Supercapacitors Based on Silver Nanowire–Activated Carbon Composites
Myounggun Kim*, **,# , Seung-Yul Lee*, **,# , Moon Jong Han***, ****, Da-Seul Kim*, **,†
 , and Ju-Hyung Kim*, **,†
, and Ju-Hyung Kim*, **,† 
*Department of Energy Systems Research, Ajou University, Suwon 16499, Korea
**Department of Chemical Engineering, Ajou University, Suwon 16499, Korea
***Department of Semiconductor & Electronic Engineering, Gachon University, Seongnam 13120, Korea
****School of Materials Science and Engineering, Georgia Institute of Technology, Atlanta, Georgia 30332, United States- 은 나노 와이어-활성탄 복합체 기반 집전체 없는 슈퍼커패시터의 간소화된 제작 공정
김명건*, **,# · 이승율*, **,# · 한문종***, **** · 김다슬*, **,†
 · 김주형*, **,†
· 김주형*, **,† 
*아주대학교 에너지시스템학과, **아주대학교 화학공학과, ***가천대학교 반도체공학과, ****조지아공과대학교 재료공학과
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
With the growing demand for lightweight and flexible energy storage systems, we developed a composite electrode for a current collector-free supercapacitor. The design combines silver nanowires (AgNWs) and activated carbon (AC) to overcome the limitations of conventional metal film current collectors. Electrochemical performance was systematically optimized by tuning the AgNW:AC ratio, with optimal results obtained at 1:24. The resulting supercapacitor exhibited a volumetric capacitance of 16.3 mF cm-3, an energy density of 1.46 μWh cm-3, and a power density of 0.13 mW cm-3 within a stable 0-0.8 V operating range. This composite electrode simplifies fabrication, reduces device weight, and offers inherent potential for mechanical flexibility. Overall, this work provides a proof-of-concept approach for the development of scalable, current collector-free supercapacitors.
경량화 및 유연성이 요구되는 에너지 저장 시스템의 수요가 증가함에 따라, 본 연구에서는 집전체가 불필요한 슈퍼커패시터용(supercapacitor) 복합 전극을 개발하였다. 제안된 전극은 은 나노와이어(AgNWs)와 활성탄(AC)을 결합하여 기존 금속 박막 집전체의 한계를 극복하고자 하였다. 전기화학적 성능은 AgNW:AC 비율을 조절하여 체계적으로 최적화하였으며, 그 결과 1:24 조성에서 최적의 성능을 확보하였다. 제작된 슈퍼커패시터는 0-0.8 V의 작동 범위 내에서 체적 정전용량 16.3 mF cm-3, 에너지 밀도 1.46 mWh cm-3, 출력 밀도 0.13 mW cm-3를 나타내었다. 이러한 복합 전극은 제작 공정을 단순화하고 소자의 무게를 줄이는 동시에 기계적 유연성 측면에서도 잠재적 가능성을 제공한다. 본 연구는 대면적 확장이 가능한 집전체 없는 슈퍼커패시터 개발을 위한 개념 증명적 접근을 제시한다.
A silver nanowire–activated carbon composite electrode was developed as a current collector-free design for supercapacitors. The optimized 1:24 ratio enables Ag nanowires to function as internal current collectors, simplifying fabrication while reducing weight and enhancing flexibility compared to conventional devices.
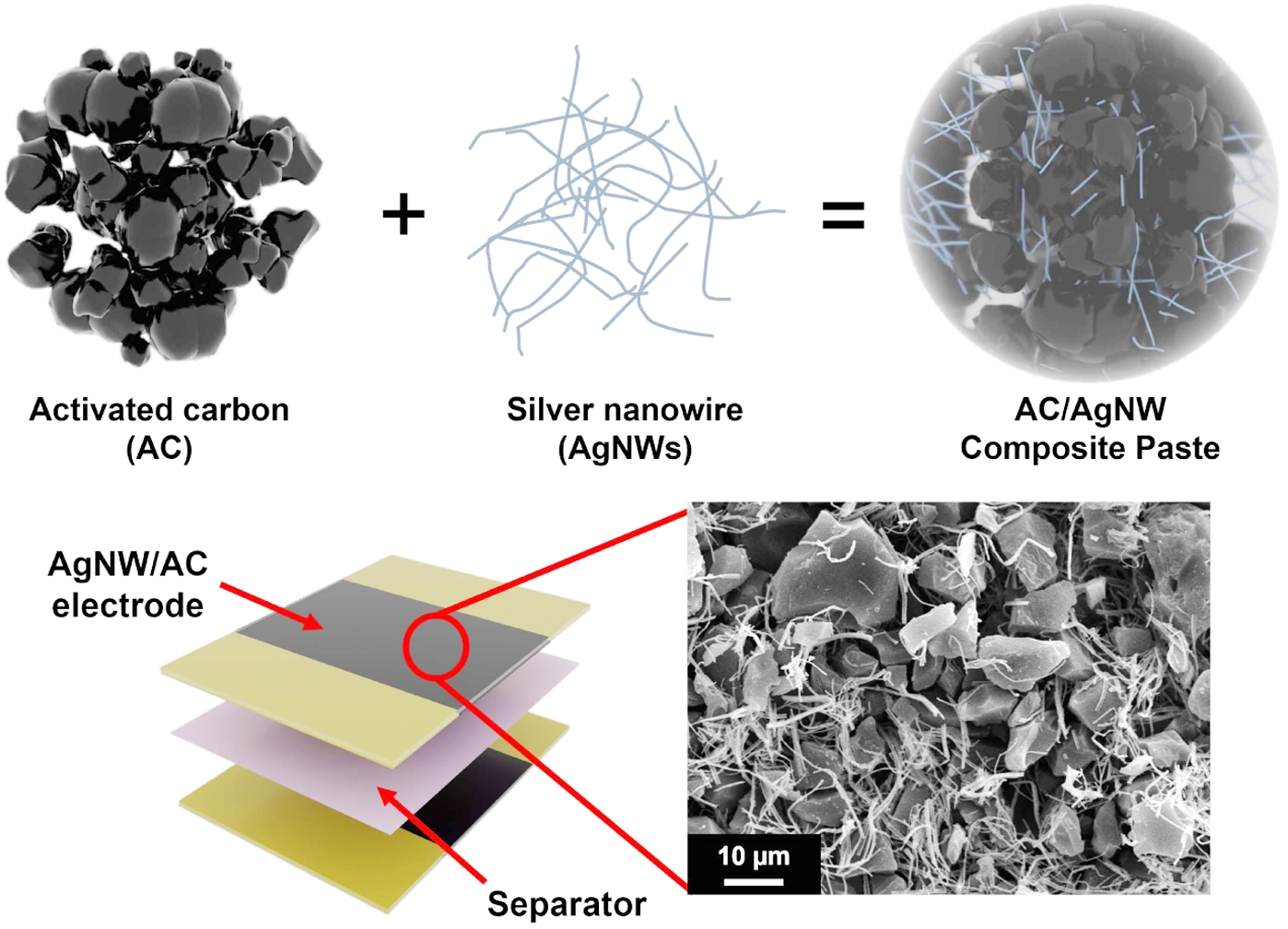
Keywords: composite electrodes, silver nanowires, activated carbon, current collector-free, supercapacitors.
This work was supported by the Ajou University research fund.
The authors declare that there is no conflict of interest.
The rapid growth of portable and wearable electronics, including Internet of Things (IoT) sensors and biomedical devices,1,2 has intensified the demand for lightweight, flexible, and high-performance energy storage systems.3-5 Lithium-ion batteries,6-8 while offering high energy density, face few limitations such as safety concerns, relatively lower power density, and long charging times.9,10 In contrast, supercapacitors based on electric double-layer capacitance (EDLCs) provide rapid charge–discharge capability, long cycle life, and high power density, making them a complementary candidate for next-generation energy storage,11 particularly where high power and durability are required.12-14
A practical limitation of conventional supercapacitors lies in their reliance on metallic current collectors, typically aluminum or nickel foils.15 These rigid and heavy components increase device weight, reduce flexibility, and add complexity to fabrication.16-19 For applications requiring portability and mechanical compliance, eliminating external current collectors or replacing them with alternatives such as conductive carbon-based materials (e.g., carbon cloth, graphite, carbon nanotube and so on) while retaining high electrical conductivity remains a significant challenge.20-24
Composite electrodes offer a promising pathway by combining conductive nanomaterials with porous carbon matrix.25-28 Activated carbon (AC) is widely used due to its large specific surface area and electrochemical stability,29-31 but its low intrinsic conductivity restricts efficient charge transport. Silver nanowires (AgNWs), with their outstanding electrical conductivity and ability to form interconnected networks, can act as built-in conductive pathways, thereby reducing or even replacing the need for external current collectors.32-34 Moreover, the one-dimensional nanowire network can impart flexibility to the electrode, as the interwoven AgNWs maintain electrical contact under mechanical deformation, complementing the structural stability provided by AC.35-37
In this work, we present a simple and cost-effective approach to fabricating AgNW/AC composite electrodes for current collector-free supercapacitors. By systematical tuning of the AgNW:AC ratio, we identify an optimal balance between electronic conductivity and ion accessibility. The optimized configuration achieves stable electrochemical performance and is expected to provide advantages in lightweight and flexible energy storage devices. This strategy highlights a practical pathway toward scalable and manufacturable supercapacitors that meet the requirements of emerging portable and wearable electronics.
Materials. Activated carbon (AC, MSP-20, SBET = 2260 m2 g-1, Korea Institute of Ceramic Engineering and Technology, KICET) was used as the active material. Silver nanowires (AgNWs, purity = 99%, diameter = 100 nm, length = 100–150 μm) were employed as a 1 wt% aqueous solution. Super P (Alfa Aesar) was used as the conductive agent. Styrene–butadiene rubber (SBR) binder and polyethylene separator were obtained from MTI Korea. Sodium carboxymethyl cellulose (CMC), lithium hexafluorophosphate (LiPF6), ethylene carbonate (EC), and ethyl methyl carbonate (EMC) were purchased from Sigma-Aldrich.
Preparation of Composite Electrode Paste and Electrolyte. The composite paste was prepared by mixing AC, Super P, and CMC/SBR binders at a weight ratio of 8:1:0.5:0.5 using a planetary mixer (THINKY). To control the coffee-ring effect and ensure uniform film formation, the amount of distilled water was adjusted to 0.5–1.2 μL per 80 mg of AC. AgNWs were subsequently added according to the specified weight percentage. The mixture was uniformly processed to achieve compositional homogeneity. A 1.0 M LiPF6 solution in ethylene carbonate/ethyl methyl carbonate (EC/EMC, 1:1 v/v) was used as the electrolyte for all electrochemical measurements.
Fabrication of Sandwich Structure Supercapacitors. A slide glass (37.5 mm × 25 mm) was used as the substrate. The prepared paste was coated using a doctor blade, dried at 80 °C for 12 h, and compressed with a roller. Two electrodes of the same size were assembled with a polyethylene separator and saturated with the electrolyte. AgNWs in the paste served as current collectors. For electrical connection to the electrochemical analyzer, a platinum wire was attached to the electrodes. The effective electrode area was 4.375 cm2, as defined by the Kapton tape on the glass substrate. This area was maintained consistently across all devices to ensure reproducibility of the electrochemical measurements.
Electrochemical Evaluations. The electrochemical characteristics of the fabricated supercapacitors were evaluated using an electrochemical workstation (ZIVE MP1, ZIVELAB). A symmetric two-electrode configuration was employed for all tests. Cyclic voltammetry (CV) was carried out within a potential window of 0.0–0.8 V at scan rates of 20 mV s-1. Based on the CV data, we evaluated the key electrochemical performance metrics using the following equations:38-40

where Ca is the volumetric capacitance [F cm-3], Ea is the volumetric energy density [Wh cm-3], and Pa is the volumetric power density [W cm-3]. The term ∫I(V)dV represents the area of the discharge curve [mA V], while ν is the scan rate [mV s-1],Vis the volume of the electrode [cm3], Du is the operational potential window [V], and Dt is the discharge time (s).
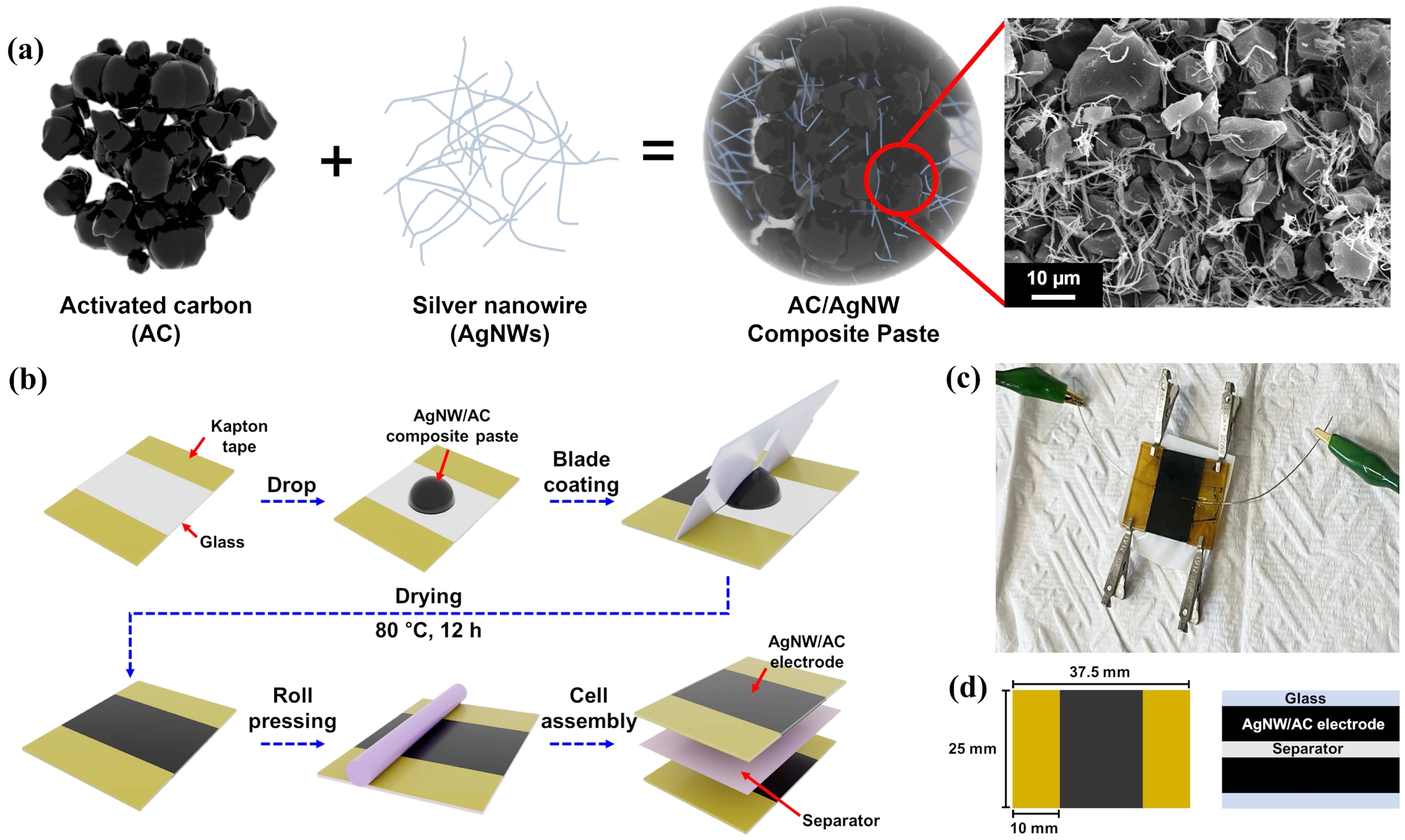
In this study, AgNW/AC composite electrodes were fabricated and assembled into current collector-free supercapacitors using a paste-based process. The overall procedure and device structure are summarized in Figure 1. As illustrated in Figure 1(a), the AgNWs form an interconnected conductive scaffold within the porous AC matrix, thereby integrating the functions of a current collector and active electrode in a single composite. This dual functionality is particularly advantageous, as it not only provides continuous electron pathways but also eliminates the need for an additional metallic thin-film current collector. The scanning electron microscopy (SEM) image confirms the uniform dispersion of AgNWs throughout the AC network, highlighting the intimate contact between the two components. The device fabrication process is illustrated in Figure 1(b), where the composite paste was coated onto the substrate using a doctor blade to ensure uniform thickness, followed by assembly with a polyethylene separator and electrolyte. Figure 1(c) presents the final symmetric cell, confirming the successful integration of the coated electrodes into a complete device. Collectively, this design strategy demonstrates how AgNWs simultaneously enhance conductivity and simplify the overall device structure. Although the present study demonstrates the concept on glass substrates, this fabrication route represents one practical strategy to reduce substrate-related constraints. By avoiding the vacuum and high-temperature steps typically required for current collector deposition, the method proceeds under mild conditions and is thus more compatible with polymer or flexible supports.
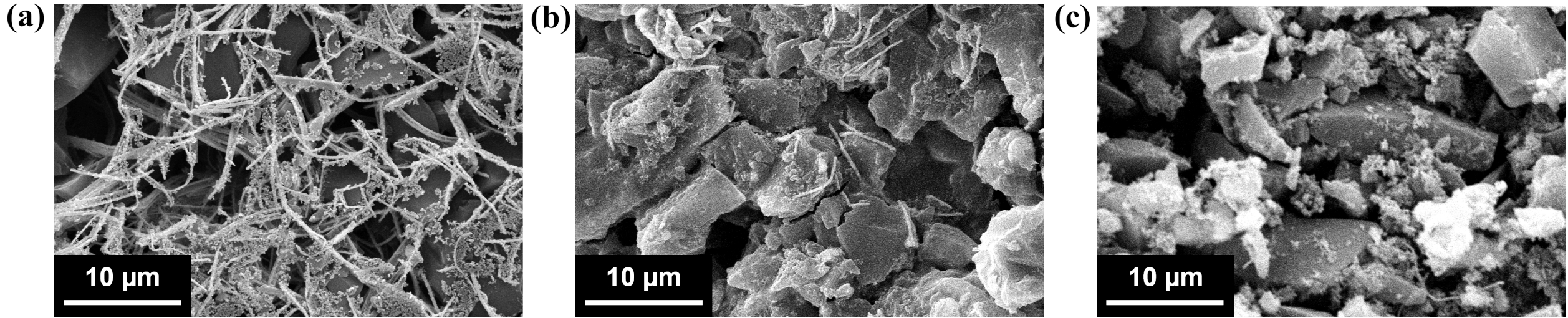
The morphology of the composites was investigated by SEM to assess how the AgNW:AC ratio influences the electrode microstructure (Figure 2). At a high AgNW:AC ratio (1:2, Figure 2(a)), the nanowires densely cover the AC particles, forming continuous bridges but partially blocking porous channels. Although the distribution is not perfectly homogeneous, this conductive matrix provides continuous electron pathways, thereby facilitating efficient charge transport to the electrochemically active AC domains. Some regions show denser nanowire bundles, likely caused by partial aggregation. Such features could reduce the uniformity of conductivity but do not fully compromise the percolating network.
At an intermediate AgNW:AC ratio (1:6, Figure 2(b)), the nanowires remain sufficiently interconnected to establish continuous conductive paths, while the porous AC matrix is less obstructed compared to the 1:2 sample. This morphology suggests an improved balance between electronic conductivity and ion accessibility, which is conducive to capacitive performance.
In contrast, at a low AgNW:AC ratio (1:32, Figure 2(c)), the nanowire density decreases markedly, leading to discontinuous conductive pathways. Many AC particles appear electronically isolated, which increases charge transfer resistance and limits the effective utilization of active sites. This structural deficiency is expected to deteriorate both conductivity and rate capability.
These observations indicate that the AgNW:AC ratio critically dictates electrode design. While excessive nanowire loading may hinder ion transport, insufficient loading compromises electronic connectivity. An intermediate ratio thus yields the most optimal structure, supporting both efficient electron conduction and ion accessibility. This correlation will be further confirmed by the subsequent electrochemical analyses.
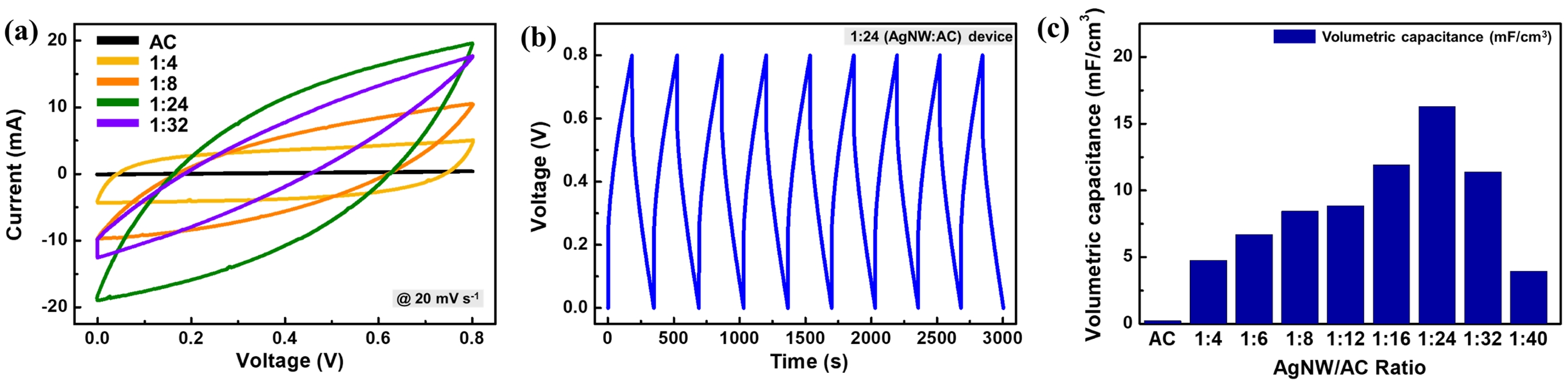
To examine the role of AgNWs as an internal current collector, cyclic voltammetry (CV) was performed on electrodes with varying ratios at a constant scan rate of 20 mV s⁻¹ (Figure 3). The CV curves (Figure 3(a)) demonstrate that the AC-only electrode exhibits negligible current response due to its limited electrical conductivity, whereas incorporation of AgNWs produces quasi-rectangular profiles characteristic of EDLC behavior. The enclosed area of the CV curves increases progressively as the AgNW:AC ratio decreases from 1:4 to 1:24, indicating both improved ion accessibility and reduced internal resistance. However, further reduction of AgNW content below 1:24 leads to a decline in the CV area, consistent with insufficient percolation of the conductive network.
Figure 3(b) shows the galvanostatic charge–discharge (GCD) curves of the optimized 1:24 electrode, recorded at a volumetric current density of 11.43 mA cm-3 over ~9 consecutive cycles. The profiles exhibit symmetric triangular shapes with a consistent IR drop of ~0.21 V, confirming capacitive behavior with finite internal resistance. This IR drop is relatively large compared with values typically reported in conventional supercapacitors,41 which can be attributed mainly to limited contact between AgNWs and AC particles within the composite structure.
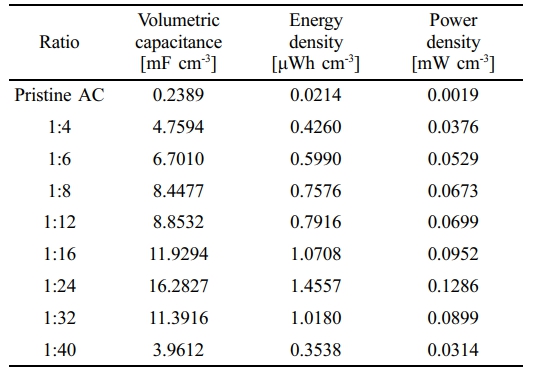
The reproducible curves indicate stable short-term operation with high coulombic efficiency, while extended cycling would be required to fully evaluate long-term durability. Figure 3(c) quantitatively summarizes the effect of the AgNW:AC ratio on electrochemical performance. The electrochemical parameters of all fabricated devices are listed in Table 1. The volumetric capacitance increases steadily as the AgNW content decreases from 1:4 to 1:24, reaching a maximum of 16.28 mF cm-3 at the 1:24 composition. At this ratio, the device also delivers an energy density of 1.46 μWh cm-3 and a power density of 0.13 mW cm-3, representing the best performance among all tested electrodes. In comparison, the AC-only electrode shows a negligible capacitance of only 0.24 mF cm-3, underscoring the essential role of AgNWs in establishing a conductive percolation network.
At ratios below 1:24, the capacitance drops sharply, indicating that the conductive network becomes discontinuous and internal resistance increases. This overall trend highlights a critical trade-off: while AgNWs are indispensable for charge transport, excessive loading reduces pore accessibility for electrolyte ions, whereas insufficient loading leaves large fractions of the electrically isolated AC. The optimum at 1:24 thus reflects the most effective balance between electronic conductivity and ionic accessibility in the current collector-free electrode design.
Overall, the 1:24 composition provides the most effective integration of AgNWs and AC, enabling efficient charge transport while maximizing the accessible surface area of the carbon matrix in a current collector-free design.
|
Figure 1 Fabrication and structure of the current collector-free AgNW/AC supercapacitor: (a) Schematic of the composite electrode materials and SEM image showing AgNW dispersion within the porous AC matrix; (b) Illustration of the electrode preparation and assembly process for the current collector-free configuration; (c) Photograph of the assembled symmetric supercapacitor; (d) Device dimensions (top view) and cross-sectional schematic illustrating the electrode–separator configuration. |
|
Figure 2 SEM images of AgNW/AC composite electrodes at different AgNW:AC ratios: (a) 1:2; (b) 1:6; (c) 1:32 |
|
Figure 3 Electrochemical performance of AgNW/AC composite electrodes at different AgNW:AC ratios: (a) CV curves at 20 mV s-1; (b) GCD curves of the 1:24 sample; (c) volumetric capacitance values extracted for all ratios. |
|
Table 1 Electrochemical Parameters of AgNW/AC Composite Electrodes at Different AgNW:AC Ratios |
This work demonstrates the feasibility of constructing current collector-free supercapacitor electrodes using AgNW/AC composites. The integrated AgNW network acts as an internal current collector while preserving the porous structure for efficient ion transport.
The collector-free configuration simplifies the device design, reduces weight, and offers inherent potential for mechanical flexibility, which is particularly advantageous for next-generation wearable and portable electronics. The fabrication process can also be carried out under mild conditions, suggesting compatibility with polymer or flexible substrates. At the same time, challenges remain, including relatively high internal resistance, which likely arises from incomplete contact between AgNWs and AC particles. This limitation could potentially be mitigated by improving electrode densification and enhancing AgNW–AC interfacial connectivity, which will be an important direction for future work. In addition, long-term cycling stability still requires validation, and comprehensive evaluations of electrical and mechanical stability will be important to advance this design toward practical applications.
Overall, this study demonstrates a proof-of-concept strategy for low-cost and flexible supercapacitors, suggesting that AgNW/AC composites hold promise for further development into practical energy storage systems.
- 1. Kim, J.; Kumar, R.; Bandodkar, A. J.; Wang, J. Advanced Materials for Printed Wearable Electrochemical Devices: A Review. Adv. Electron. Mater. 2017, 3, 1600260.
-

- 2. Cima, M. J. Next-generation Wearable Electronics. Nat. Biotechnol. 2014, 32, 642-643.
-

- 3. Choi, S.; Lee, H.; Ghaffari, R.; Hyeon, T.; Kim, D. H. Recent Advances in Flexible and Stretchable Bio‐electronic Devices Integrated with Nanomaterials. Adv. Mater. 2016, 28, 4203-4218.
-

- 4. Fang, W.; Zhao, J.; Zhang, W.; Chen, P.; Bai, Z.; Wu, M. Recent Progress and Future Perspectives of flexible Zn-air batteries. J. Alloys Compd. 2021, 869, 158918.
-

- 5. Hassan, M.; Abbas, G.; Li, N.; Afzal, A.; Haider, Z.; Ahmed, S.; Xu, X.; Pan, C.; Peng, Z. Significance of Flexible Substrates for Wearable and Implantable Devices: Recent Advances and Perspectives. Adv. Mater. Technol. 2022, 7, 2100773.
-

- 6. Li, M.; Lu, J.; Chen, Z.; Amine, K., 30 years of Lithium‐ion Batteries. Adv. Mater. 2018, 30, 1800561.
-

- 7. Kim, T.; Song, W.; Son, D.-Y.; Ono, L. K.; Qi, Y. Lithium-ion Batteries: Outlook on Present, Future, and Hybridized Technologies. J. Mater. Chem. A 2019, 7, 2942-2964.
-

- 8. Manthiram, A. An Outlook on Lithium Ion Battery Technology. ACS Cent. Sci. 2017, 3, 1063-1069.
-

- 9. Zhang, H.; Zhang, J.; Gao, X.; Wen, L.; Li, W.; Zhao, D. Advances in Materials and Structures of Supercapacitors. Ionics 2022, 28, 515-531.
-

- 10. Khan, M. I.; Gilani, R.; Hafeez, J.; Ayoub, R.; Zahoor, I.; Saira, G. Advantages and Disadvantages of Lithium-ion Batteries. In NanostructuredLithium-Ion Battery Materials: Synthesis, Characterization, and Applications; Thomas, S., Gueye, A. B., Savadogo, O., Maria, H. J., Eds.; Elsevier: Amsterdam, 2025; pp 47-64.
-

- 11. Olabi, A. G.; Abbas, Q.; Al Makky, A.; Abdelkareem, M. A. Supercapacitors as Next Generation Energy Storage Devices: Properties and Applications. Energy 2022, 248, 123617.
-

- 12. Iro, Z. S.; Subramani, C.; Dash, S. A Brief Review on Electrode Materials for Supercapacitor. Int. J. Electrochem. Sci. 2016, 11, 10628-10643.
-

- 13. Raza, W.; Ali, F.; Raza, N.; Luo, Y.; Kim, K.-H.; Yang, J.; Kumar, S.; Mehmood, A.; Kwon, E. E. Recent Advancements in Supercapacitor Technology. Nano Energy 2018, 52, 441-473.
-

- 14. Karthikeyan, S.; Narenthiran, B.; Sivanantham, A.; Bhatlu, L. D.; Maridurai, T. Supercapacitor: Evolution and review. Mater. Today: Proc. 2021, 46, 3984-3988.
-

- 15. Blomquist, N.; Wells, T.; Andres, B.; Bäckström, J.; Forsberg, S.; Olin, H. Metal-free Supercapacitor with Aqueous Electrolyte and Low-cost Carbon Materials. Sci. Rep. 2017, 7, 39836.
-

- 16. Baskakov, S.; Baskakova, Y.; Lyskov, N.; Dremova, N.; Irzhak, A.; Kumar, Y.; Michtchenok, A.; Shulga, Y. Fabrication of Current Collector Using a Composite of Polylactic Acid and Carbon Nano-material for Metal-free Supercapacitors with Graphene Oxide Separators and Microwave Exfoliated Graphite Oxide Electrodes. Electrochim. Acta 2018, 260, 557-563.
-

- 17. Liu, J.; Mirri, F.; Notarianni, M.; Pasquali, M.; Motta, N. High Performance All-carbon Thin Film Supercapacitors. J. Power Sources 2015, 274, 823-830.
-

- 18. Yu, J.; Wu, J.; Wang, H.; Zhou, A.; Huang, C.; Bai, H.; Li, L. Metallic Fabrics as the Current Collector for High-performance Graphene-based Flexible Solid-state Supercapacitor. ACS Appl. Mater. Interfaces 2016, 8, 4724-4729.
-

- 19. Tanwilaisiri, A.; Xu, Y.; Harrison, D.; Fyson, J.; Arier, M. A Study of Metal Free Supercapacitors Using 3D Printing. Int. J. Precis. Eng. Manuf. 2018, 19, 1071-1079.
-

- 20. Ramadoss, A.; Wong, K. K.; Swain, N.; Mohanty, A.; Kirubavathi, K.; Selvaraju, K.; Schmidt Mende, L. Flexible, Lightweight, and Ultrabendable RuO2–MnO2/graphite Sheets for Supercapacitors. Energy Fuels 2022, 36, 11194-11204.
-

- 21. Jung, S. Y.; Nah, B. R.; Cho, I. W.; Choi, J.; Yang, M. Improving Wettability and Adhesion of Carbon Cloth with Polydopamine for a Flexible Supercapacitor. Carbon Lett. 2022, 32, 329-337.
-

- 22. Chen, B.; Wong, W.-Y. Introducing a Redox-active Ferrocenyl Moiety Onto a Polythiophene Derivative Towards High-performance Flexible All-solid-state Symmetric Supercapacitors. J. Mater. Chem. A 2022, 10, 7968-7977.
-

- 23. Abdisattar, A.; Yeleuov, M.; Daulbayev, C.; Askaruly, K.; Tolynbekov, A.; Taurbekov, A.; Prikhodko, N. Recent Advances and Challenges of Current Collectors for Supercapacitors. Electrochem. Commun. 2022, 142, 107373.
-

- 24. Guo, G.; Shen, L.; Li, X.; Cao, Y.; Sun, Y.; Xiong, Z. Tunable Reduction Degree of Stacked Lamellar rGO Film for Application in Flexible All-solid-state Supercapacitors. Diam. Relat. Mater. 2020, 106, 107845.
-

- 25. Hu, M.; Zhang, H.; Hu, T.; Fan, B.; Wang, X.; Li, Z. Emerging 2D MXenes for Supercapacitors: Status, Challenges and Prospects. Chem. Soc. Rev. 2020, 49, 6666-6693.
-

- 26. Wang, F.; Wu, X.; Yuan, X.; Liu, Z.; Zhang, Y.; Fu, L.; Zhu, Y.; Zhou, Q.; Wu, Y.; Huang, W. Latest Advances in Supercapacitors: From New Electrode Materials to Novel Device Designs. Chem. Soc. Rev. 2017, 46, 6816-6854.
-

- 27. Panda, S.; Deshmukh, K.; Pasha, S. K.; Theerthagiri, J.; Manickam, S.; Choi, M. Y. MXene Based Emerging Materials for Supercapacitor Applications: Recent Advances, Challenges, and Future Perspectives. Coord. Chem. Rev. 2022, 462, 214518.
-

- 28. Chen, Y.; Yang, H.; Han, Z.; Bo, Z.; Yan, J.; Cen, K.; Ostrikov, K. K. MXene-based Electrodes for Supercapacitor Energy Storage. Energy Fuels 2022, 36, 2390-2406.
-

- 29. Frackowiak, E. Carbon Materials for Supercapacitor Application. Phys. Chem. Chem. Phys. 2007, 9, 1774-1785.
-

- 30. Teo, E. Y. L.; Muniandy, L.; Ng, E.-P.; Adam, F.; Mohamed, A. R.; Jose, R.; Chong, K. F. High Surface Area Activated Carbon from Rice Husk as a High Performance Supercapacitor Electrode. Electrochim. Acta 2016, 192, 110-119.
-

- 31. Li, B.; Dai, F.; Xiao, Q.; Yang, L.; Shen, J.; Zhang, C.; Cai, M. Nitrogen-doped Activated Carbon for a High Energy Hybrid Supercapacitor. Energy Environ. Sci. 2016, 9, 102-106.
-

- 32. Xu, F.; Zhu, Y. Highly Conductive and Stretchable Silver Nanowire Conductors. Adv. Mater. 2012, 24, 5117-5122.
-

- 33. Hu, L.; Kim, H. S.; Lee, J.-Y.; Peumans, P.; Cui, Y. Scalable Coating and Properties of Transparent, Flexible, Silver Nanowire Electrodes. ACS Nano 2010, 4, 2955-2963.
-

- 34. Tan, D.; Jiang, C.; Li, Q.; Bi, S.; Song, J. Silver Nanowire Networks with Preparations and Applications: A Review. J. Mater. Sci.: Mater. Electron. 2020, 31, 15669-15696.
-

- 35. Yu, C.; Fang, S.; Chen, Y.; Wu, X.; Xu, R.; Chen, Y.; Feng, Y.; Shi, B.; Li, Q.; Cao, Z. High-performance Flexible All-solid-state Supercapacitors Integrated with Self-healing Hydrogel Electrolyte and Silver Nanowire Electrodes. Mater. Today Nano 2025, 30, 100619.
-

- 36. Wang, B.; Zhao, X.; Liang, J.; Liu, J.; Yang, Y.; Zhang, M.; Yu, H.; Li, J.; Tong, Y.; Tang, Q. Microwave-welded and Photopolymer-embedded Silver Nanowire Electrodes for Skin-like Supercapacitors. ACS Appl. Energy Mater. 2022, 5, 10490-10500.
-

- 37. Kim, T.; Park, C.; Samuel, E. P.; An, S.; Aldalbahi, A.; Alotaibi, F.; Yarin, A. L.; Yoon, S. S. Supersonically Sprayed Washable, Wearable, Stretchable, Hydrophobic, and Antibacterial rGO/AgNW Fabric for Multifunctional Sensors and Supercapacitors. ACS Appl. Mater. Interfaces 2021, 13, 10013-10025.
-

- 38. Zhao, F.; Zheng, D.; Liu, Y.; Pan, F.; Deng, Q.; Qin, C.; Li, Y.; Wang, Z. Flexible Co(OH)2/NiOxHy@ Ni Hybrid Electrodes for High Energy Density Supercapacitors. Chem. Eng. J. 2021, 415, 128871.
-

- 39. Cao, Y.; Zhang, J.; Yang, W.; Li, Y.; Chen, H.; Hao, Q.; Ma, X. Handy Preparation of a Carbon-Ni/NiO/Ni (OH)2 Composite and Its Application in High-performance Supercapacitors. Electrochim. Acta 2024, 497, 144618.
-

- 40. Tao, Y.; Xie, X.; Lv, W.; Tang, D.-M.; Kong, D.; Huang, Z.; Nishihara, H.; Ishii, T.; Li, B.; Golberg, D. Towards Ultrahigh Volumetric Capacitance: Graphene Derived Highly Dense But Porous Carbons for Supercapacitors. Sci. Rep. 2013, 3, 2975.
-

- 41. Dissanayake, K.; Kularatna-Abeywardana, D. A Review of Supercapacitors: Materials, Technology, Challenges, and Renewable Energy Applications. J. Energy Storage 2024, 96, 112563.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2024 Impact Factor : 0.6
- Indexed in SCIE
 This Article
This Article
-
2026; 50(1): 161-166
Published online Jan 25, 2026
- 10.7317/pk.2026.50.1.161
- Received on Sep 8, 2025
- Revised on Oct 7, 2025
- Accepted on Oct 11, 2025
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
- Conflict of Interest
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Da-Seul Kim*, ** , and Ju-Hyung Kim*, **
-
*Department of Energy Systems Research, Ajou University, Suwon 16499, Korea
**Department of Chemical Engineering, Ajou University, Suwon 16499, Korea - E-mail: kimda313@ajou.ac.kr, juhyungkim@ajou.ac.kr
- ORCID:
0009-0004-0828-3866, 0000-0002-0925-3368








 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.