- Layer-by-layer Adsorption of Glucose Oxidase on Graphene-polystyrene Composite for Glucose Detection
Department of Cosmetics and Biotechnology, Semyung University, Jecheon 27136, Korea
- 글루코오스 감지를 위한 그래핀-폴리스티렌 복합체 위에 층 쌓기 방법에 의한 글루코오스 산화 효소의 흡착
세명대학교 화장품 생명공학과
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
An electrically conductive graphene-polystyrene composite was prepared by mixing an exfoliated phenylisocyanate-treated graphite oxide sheet with polystyrene, followed by chemical reduction with dimethylhydrazine. Glucose oxidase was immobilized on this composite via electrostatic interaction using a layer-by-layer adsorption technique, with poly(diallyldimethylammonium chloride) as the counter-ionic material. A quartz crystal microbalance was used to estimate the amount of the adsorbed layer material. An amperometric glucose biosensor, fabricated with the glucose oxidase-immobilized graphene-polystyrene composite as a working electrode, showed significantly improved efficacy for measuring glucose concentration.
Phenylisocyanate로 처리된 층 분리된 graphite oxide와 폴리스티렌을 혼합하고 dimethylhydrazine으로 환원시켜 전도성을 갖는 그래핀-폴리스티렌 복합체를 제조하였다. 글루코오스 산화 효소는 poly(diallyldimethylammonium chloride)를 반대편 상대 물질로 사용하고, 층 쌓기 흡착 방법을 이용해서 정전기적인 인력으로 이 복합체 위에 고정되었다. 층을 형성하는 물질의 양은 quartz crystal microbalance를 이용해서 측정하였다. 그래핀-폴리스티렌 복합체에 고정된 글루코오스 산화 효소를 이용해서 형성된 전류 측정 방식의 글루코오스 센서는 글루코오스 농도 측정에 상당히 우수한 결과들을 보여 주었다.
Glucose oxidase was immobilized on the graphene-polystyrene composite using a layer-by-layer adsorption technique, with poly(diallyldimethylammonium chloride) as the counter-ionic material. An amperometric glucose biosensor, fabricated with this composite as a working electrode, showed significantly improved efficacy for measuring glucose concentration.
Keywords: glucose, biosensor, graphene, composite, electrostatic layer-by-layer adsorption.
The authors declare that there is no conflict of interest.
Graphene composites are recognized as a very useful material, and many research groups have recently attempted their use in various fields.1 Graphene is a single sheet of graphite, exhibiting a unique structure and extraordinary mechanical, thermal, and electrical properties.2 When graphene is incorporated into a polymer, these properties can be conferred to the polymeric material.3-7 Graphene-based polymer composites have an extraordinarily low electrical percolation threshold due to the high conductivity of graphene.6 These composites have excellent thermal and mechanical properties compared to other carbon-based composites due to the intense interactions between graphene and the polymeric materials.3 The composites can be used as conductors due to the highly conductive nature of graphene and its good blendability with polymers.8
Graphite oxide can be formed by the oxidation of graphite. Graphene oxide is a single sheet of graphite oxide. In contrast to pristine graphene, the surface of graphene oxide contains hydroxyl, epoxide, carbonyl, and carboxyl functional groups.9 The graphene oxide sheet is hydrophilic due to these functional groups, and therefore, graphite oxide can swell and finally be dispersed as graphene oxide in water. A stable aqueous dispersion of graphene oxide can be formed by the ultrasonication of graphite oxide in water, in which graphite oxide is fully exfoliated to graphene oxide sheets.10 Graphene oxide can be used as a filler for polymeric materials.11
An amperometric biosensor is a device for determining the concentration of a biological analyte using an electrical signal. Biosensors are very useful devices for many applications, for example, food, pharmacology, pollution monitoring, agricultural processing, and health care, etc.12 During the previous thirty years, many attempts have been made to develop more sensitive and lower-cost glucose sensors because measuring of the glucose level in blood is very important in clinical application due to the potential for fatality.13
Equations (1) and (2) show the reactions that occur in a glucose biosensor during determination of the glucose concentration.
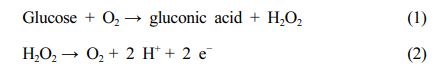
Eq. (1) represents the oxidation of glucose by glucose oxidase (GOx). The hydrogen peroxide formed through the reaction corresponding to eq. (1) can be detected by measuring the current change (in amperes) caused by the oxidation reaction (eq. (2)). The glucose concentration can be then calculated from the hydrogen peroxide concentration.
Many enzyme immobilization methods can be used to form biosensors, including covalent bonding, the crosslinking method, sol-gel reaction, and entrapment via electrostatic interaction. All of the methods have their own merits and demerits, and many researches have attempted to develop more sensitive and lower-cost sensors using various immobilization methods.14
Layer-by-layer structures formed by the alternate adsorption of a charged protein and oppositely charged materials have been reported.15 The electrostatic interaction in this structure is an important force for maintaining the layer-by-layer structure. Several proteins can be assembled in the layer-by-layer structure with many opposite charged materials (for example polyionic materials,16 inorganic nanoparticles,17 and inorganic materials by sol-gel processes,18 etc.).
In this study, a glucose biosensor is fabricated using a graphene-polystyrene composite. GOx is immobilized on this material via electrostatic interaction using a layer-by-layer adsorption technique.
Materials and Instruments. Polystyrene (Mw=350000, Mn=170000), K2S2O8, P2O5, KMnO4, graphite, poly(diallyldimethylammonium chloride) (PDDA), hydrogen peroxide, glucose, dimethylhydrazine, glucose oxidase (GOx), sulfuric acid, 2-mercaptoethanol, phenylisocyanate, dimethylformamide (DMF), and ethanol were obtained from Sigma-Aldrich Co. (St. Louis, MO, USA). SKC SY51 polyester film (thickness: 150 mm), which has a micro-void structure, was used as a base material to form the biosensor. A 0.500 M glucose solution was prepared daily and it was stored for 12 h to reach the mutarotational equilibrium state prior to use. The layer thickness was determined using a quartz crystal microbalance (QCM) (USI System, Fukuoka, Japan) and quartz resonators (USI System, Fukuoka, Japan). An Ivium potentiostat (Ivium Technologies, Netherlands) was used to detect the current change (in amperes) during the glucose concentration detection. An IR Spirit-T (Shimadzu, Tokyo, Japan) instrument was used to obtain the infrared (IR) spectra.
Preparation of Graphite Oxide. Graphite powder (1.00 g) was added to 4.0 mL of H2SO4 in a 250 mL round-bottomed flask, and the temperature was raised to 80 °C. K2S2O8 (0.8 g) and 0.8 g of P2O5 were added to this mixture at 80 °C with continuous stirring, and the reaction was followed for 6 h at 80 °C. Thereafter, 170 mL of water was added to this mixture, followed by filtration. The washing process was repeated until the pH of the filtrate became neutral. The product was dried at 35 °C in an oven for 18 h.
H2SO4 (40 mL) was added very slowly to this product in a 500 mL round-bottomed flask that was positioned in an ice-bath. KMnO4 (5.00 g) was added slowly at a temperature of 10 °C, and the color of the solution changed from black to dark green. The reaction was continued at 35 °C for 2 h and the color of the solution changed from dark green to dark brown. Thereafter, 80 mL of water was slowly added at a temperature of 50 °C, and the reaction was continued for 2 h at 50 °C. The color of the solution changed from dark brown to purple. Thereafter, 240 mL of water was slowly added and 4.0 mL of 30% H2O2 was added very slowly for 15 min. The reaction was continued for 1 h at 80 °C, and the color of the solution changed from purple to brown. The product was washed with 400 mL of 10% HCl and then washed with 500 mL of water using a centrifugation method in which the upper solution was repeatedly removed. Finally, the product was dried under vacuum at 60 °C for 24 h.
Synthesis of Graphene-Polystyrene Composite. Graphite oxide (0.200 g) was suspended in 20 mL of anhydrous DMF and reacted with 0.500 g (4.20 mmol) of phenyl isocyanate for 24 h; the product was collected by filtration with a glass funnel. Phenyl isocyanate-treated graphite oxide was subjected to ultrasonic exfoliation in 200 mL of DMF to produce a stable dispersion. Polystyrene (4.00 g) was added to this dispersion and dissolved with stirring. This material was reduced with 2.00 mL of dimethylhydrazine at 80 °C for 24 h. Coagulation of the polymer composites was accomplished by adding the DMF solution dropwise into a 10-fold larger volume of vigorously stirred methanol. The coagulated composite powder was isolated by filtration and then washed with 200 mL of methanol. The product was dried at 120 °C under vacuum for 12 h.
Coating of Graphene-Polystyrene Composite onto Polyester Film. The coating solution was prepared and deposited as follows. The graphene-polystyrene composite (1.00 g) was dissolved in 20 mL of DMF. The solution was magnetically stirred for 18 h to produce the solution for the coating process. The polyester film (5 cm × 1 cm) was dipped into the solution and then removed. This film was dried at 20 °C for 24 h and finally dried again in a vacuum oven at 120 °C for 12 h.
Immobilization of GOx by Layer-by-Layer Adsorption. The film that was coated with the graphene-polystyrene composite was immersed in a 2.0 wt% PDDA aqueous solution (20 mL) for 30 min, then rinsed with water twice. The GOx layer was deposited by placing the film into 20 mL of 2.0 mg/mL GOx in a pH 7 phosphate buffer solution for 60 min. The film was then rinsed with water for 30 sec, followed by a second rinse with water. When many layers of GOx were required, the GOx and PDDA adsorption processes were alternately repeated.
QCM Experiment. QCM measurements were used to estimate the change in the layer thickness during each adsorption step. In the measurement process, the resonator was immersed to allow adsorption in the solution, and was dried with nitrogen gas; the change in frequency during the adsorption process was measured. The quartz resonators were covered with gold on all the faces; the resonance frequency was 9 MHz. The surface roughness factor of the resonator was 1.1±5%, which was estimated by scanning electron microscopy.19 The reproducibility was ±2 Hz. The mass increase [M(g)] on the resonator during adsorption was estimated by calculating the frequency shift [∆F(Hz)] in the QCM using the Sauerbery equation.20 The value can be calculated considering the resonator characteristics: ∆F= -1.832×108 M/A, where A=0.16±0.01 cm2, corresponding to the surface area of the resonator. A 1 Hz change in DF corresponds to a final mass change of 0.87 ng. The thickness of the adsorbed layer (d) on both sides of the resonator was estimated using the following eq.:20 d(nm)≈-0.16 DF(Hz). The density of the adsorbed PDDA film and the GOx film was assumed to be 1.2±0.1 g/cm3 and 1.3±0.1 g/cm3, respectively.
Measurement of Adsorbed Amount Using QCM Technique. Formation of the adsorbed layer was evaluated and the resonator frequency was measured in sequence as follows: the gold surface of the resonator was cleaned with piranha solution (hydrogen peroxide and sulfuric acid (1:3, v/v)). The surface was washed with water, then dried using nitrogen gas, and the frequency of the resonator was estimated. The resonator was immersed in 5.0 mL of 10 mM 2-mercapotoethanol in ethanol for 24 h. The resonator was then placed in water for 30 min. The surface was rinsed three times with water and dried with nitrogen gas. The frequency change was estimated. After measuring the frequency change, the resonator was immersed in 2 wt% PDDA aqueous solution for 30 min. The PDDA-coated resonator was rinsed with water twice, and was dried using nitrogen gas. The frequency change was then measured. The resonator coated with PDDA was immersed in 3.0 mL of 2.0 mg/mL GOx solution (in pH 7 phosphate buffer solution) for 60 min to immobilize GOx. The resonator was rinsed with water twice, and was dried using nitrogen gas. The frequency change was then estimated. Numerous GOx and PDDA layers were deposited by repeating the GOx immobilizing and the PDDA coating processes alternately. In each step, the resonator was dried using nitrogen gas, and the frequency change was estimated.
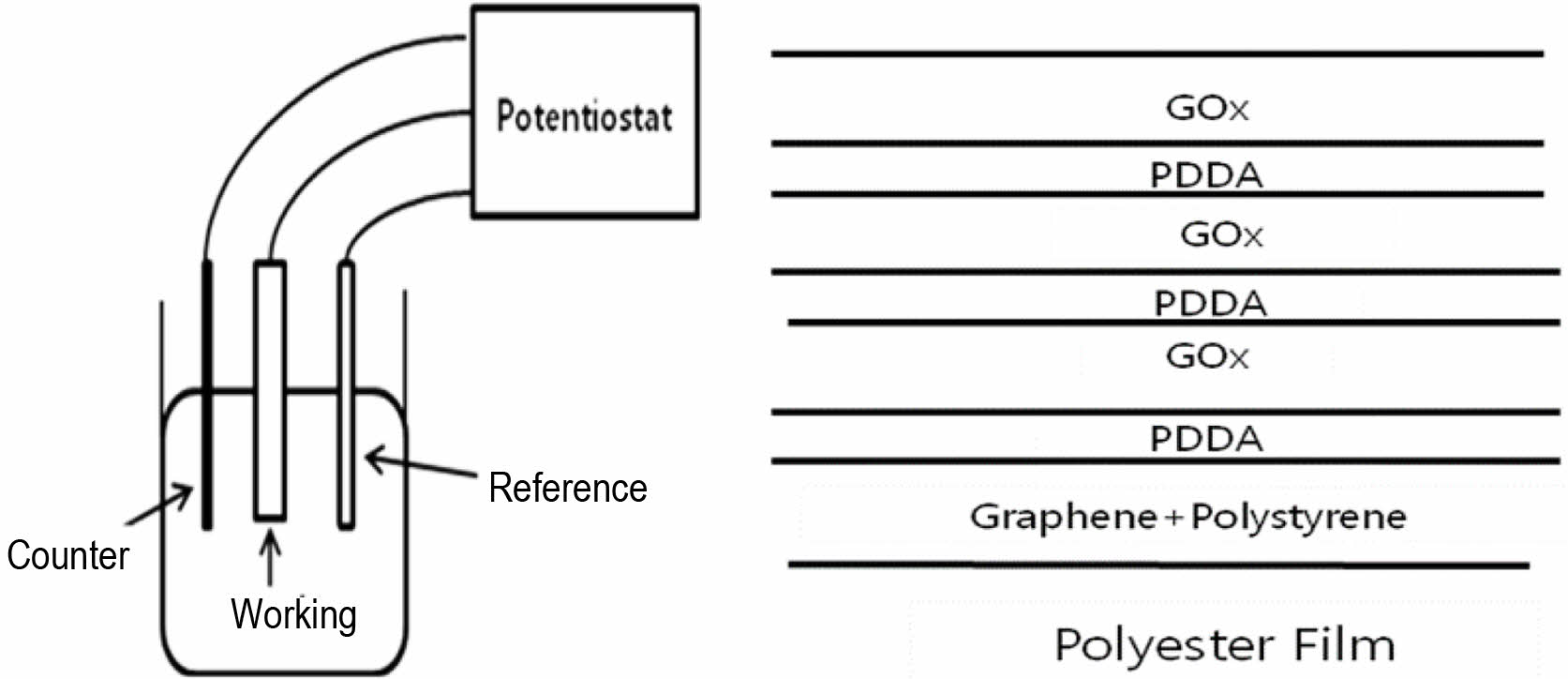
Measurement of Glucose Concentration. The working electrode for the glucose biosensor was fabricated using the film on which GOx had been immobilized with the layer-by-layer adsorption method. The working area in the film was 4 cm×1 cm×2. All electrochemical studies were conducted using 3-electrode system in a standard one-compartment cell. A Ag/AgCl (3 M KCl) electrode was used as the reference electrode and a platinum wire (10 cm) was used as the counter electrode. A schematic of the sensor is shown in Figure 1. A glucose stock solution was prepared and allowed 24 h for the equilibrium of α and β anomers.
|
Figure 1 Schematic illustrations of sensor and working electrode. |
Synthesis of the Graphene-Polystyrene Composite. To synthesize the graphene-polystyrene composite, graphene must be able to mix freely with polystyrene. However, graphite could not be exfoliated at a stretch as graphenes which is monolayer state of graphite. In this study, the graphite was oxidized to graphite oxide, which could be exfoliated, and phenyl groups were introduced into the graphene oxide. The graphite oxide is exfoliated as phenylated grapheme oxide and mixed with polystyrene to form the graphene-polystyrene composite.
It is known that graphite can be oxidized using many different oxidizing methods (9-14). In this study we referred to several papers regarding the oxidation of graphite, and the graphite was finally oxidized using several methods consecutively. The oxidation of graphite was confirmed by checking the color change and examining functional group in the IR spectrum, which were formed during oxidation reactions. The formed graphite oxide was dissolved in DMF, and reacted with phenylisocyanate to form the phenylated graphene oxide which contained numerous pendant phenyl groups as side chains. This phenylated graphene oxide mixes very well with polystyrene which also contains many phenyl groups; therefore, polystyrene was added to the solution of phenylated graphene oxide. In this solution, the reduction was conducted using dimethylhydrazine to finally form the graphene-polystyrene mixture. As a result, the graphene in this mixture was not pristine grapheme, but rather graphene containing numerous phenyl groups.
Immobilization of GOx onto the Graphene-Polystyrene Composite. For immobilization of GOx onto the graphene-polystyrene composite, the first step is the coating of the graphene-polystyrene composite onto a polyester film. The formed composite was dissolved in DMF, and the dip coating was conducted in this solution.
Using the polyester film coated with the graphene-polystyrene composite, the alternative coating was conducted with PDDA and GOx, respectively. The composite-coated film was dipped into the PDDA solution to form a single layer of PDDA on the graphene-polystyrene composite; then, the formed PDDA layer was dipped into the GOx solution to form the single layer of GOx.
The interaction between the graphene-polystyrene composite layer and the first PDDA layer is derived from the remaining oxidized functional groups in the graphene and the cations in PDDA. Even though the reaction of graphite oxide with phenylisocyanate and the reduction of the graphite oxide were conducted with dimethylhydrazine, some amount of the oxidized functional groups still remained in the graphene. These oxidized functional groups in the graphene can be reacted with the cation of PDDA, and the interaction between the PDDA layer and the GOx layer is derived from the cation in PDDA and the anion at the surface of GOx. It has been reported that GOx contains numerous anionic functional groups on its surface at natural pH. This counter ionic interaction can drive to produce the layer-by-layer adsorption, and >10 alternative layers of PDDA and GOx can be formed using this layer-by-layer adsorption method.
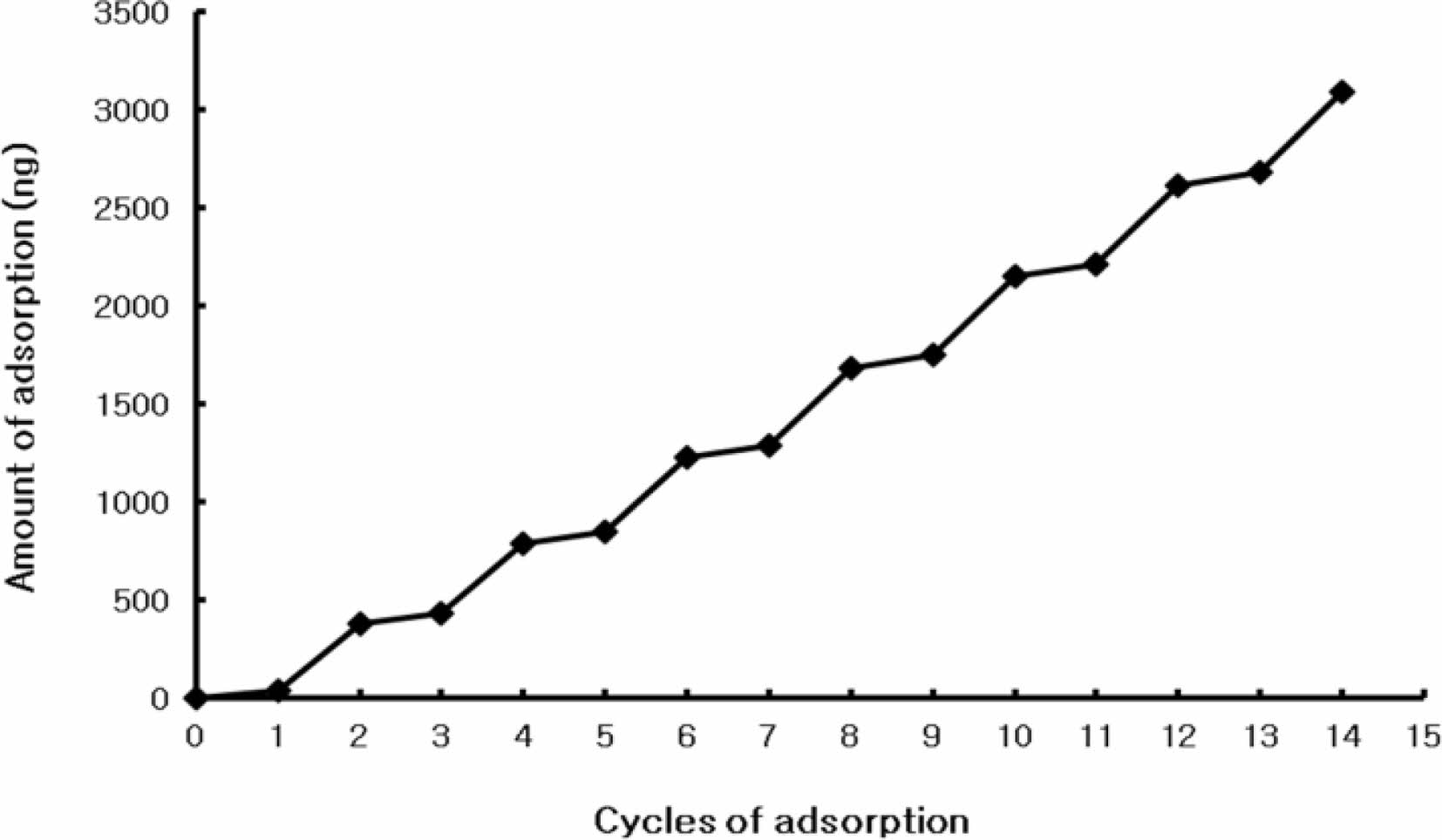
Because it is very difficult to directly estimate the amount of adsorption on the film, it was attempted to estimate the adsorption on the film using QCM. To use QCM, the resonator must be used in the estimation process. The surface of the resonator was coated with gold. The self-assembled monolayer was formed by dipping the resonator in the solution of 2-mercaptoethanol. The thiol functional group has a strong interaction with gold to form the self-assembled monolayer on surface of gold. The 2-mercaptoethanol gets one thiol group and one hydroxyl group. After the self-assembled monolayer was formed, all of the hydroxyl groups were positioned at the surface of the self-assembled monolayer. These hydroxyl groups can strongly interact with the ammonium cations in PDDA. When the polyester film which got the 2-mercatoethanol self-assembled monolayer was dipped into the PDDA solution, the monolayer of PDDA was positioned on the self-assembled monolayer. This polyester film coated with PDDA was then dipped into the GOx solution to form the GOx layer on the PDDA monolayer. We tried to dip again using these two solutions (PDDA solution and GOx solution) in order to form the alternative layer of PDDA and GOx. Every time the layer-by-layer adsorption was completed, the frequency reduction was recorded. Figure 2 showed the estimates of the adsorption amount and the thickness on the resonator. Even though the resonator did not have the exact same conditions as the surface of the graphene-polystyrene composite, similar conditions could be achieved after the basic single monolayer was formed; therefore, the amount of adsorption and layer thickness could be estimated accurately from the QCM data.
Figure 2 shows that there was a 49-73 Hz decrease in the frequency in the case of the PDDA layer, indicating that 43-64 ng or 7.8-12 Å (thickness) of PDDA was adsorbed during a single adsorption step. There was a 391-472 Hz decrease in the frequency upon adsorption of the GOx layer, indicating that 340-411 ng or 63-76 Å (thickness) of GOx was adsorbed during a single adsorption step.
Glucose Concentration Measurement. The phenylated graphene-polystyrene composite was coated on the polyester film. Thereafter, PDDA and GOx were alternately coated onto this film to form the layers. The concentration of glucose in solution was measured by using the layer-by-layer coated film.
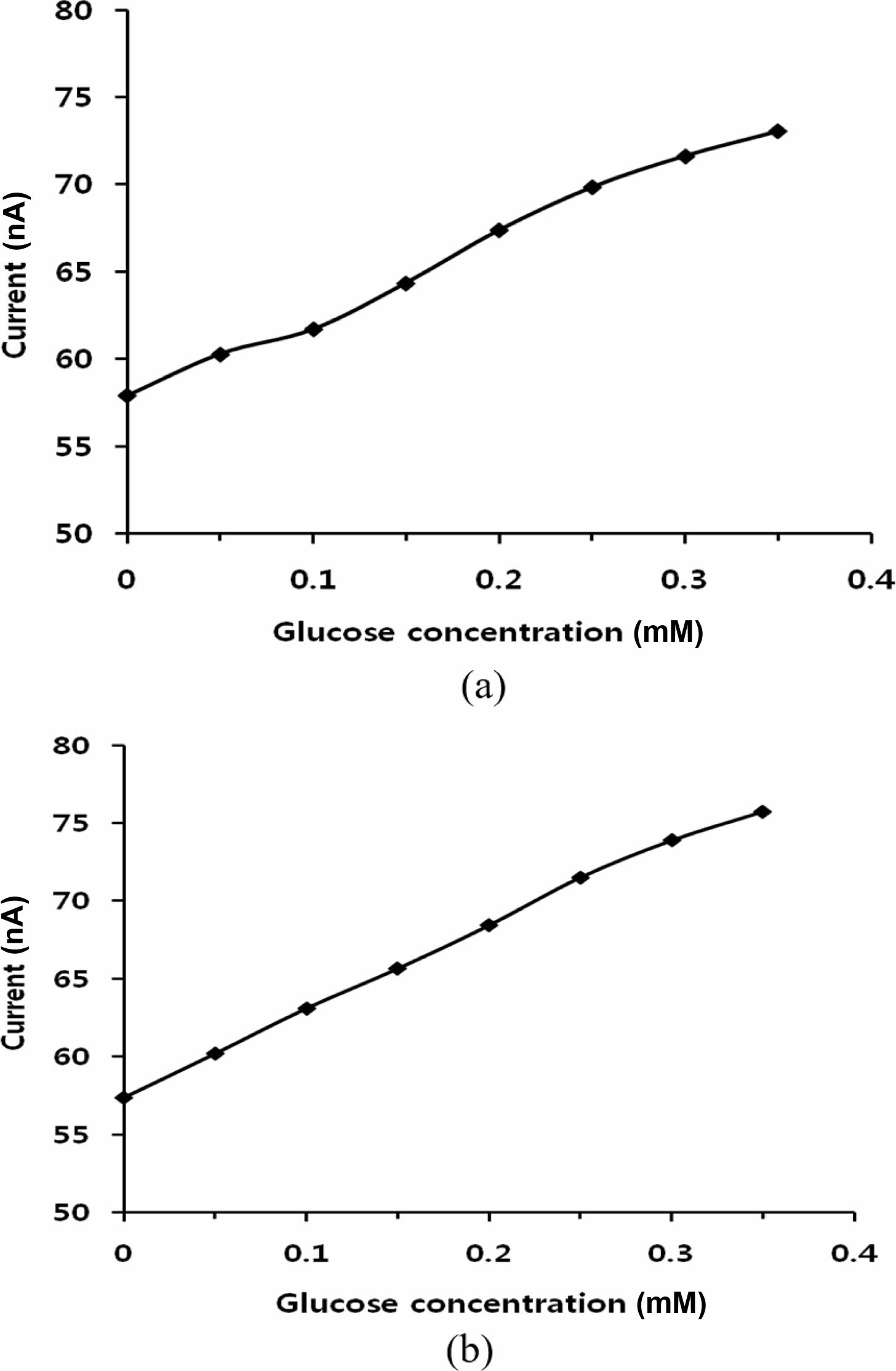
A glucose biosensor was fabricated with the GOx-immobilized film. The GOx-immobilized film, which was used as the working electrode, was prepared by either two or four alternate cycles of GOx and PDDA adsorption. Glucose stock solution was added to increase the concentration by 0.05 mM consecutively every 50 sec under 0.500 V charging. Figure 3 shows the current increase with increasing glucose concentration. Figure 3(a) shows the data obtained using the film on which GOx had been immobilized in two cycles. Figure 3(b) shows the data obtained using the film in which GOx had been immobilized four times. Figure 3(b) shows more accurate data with less deviation than Figure 3(a). These results were not significantly different from those obtained using the films with >4 layer. This similarity means that four layers are sufficient to fabricate the sensor for glucose detection.
|
Figure 2 Frequency shift according to layer-by-layer adsorption |
|
Figure 3 Amperometric detection with consecutive 0.05 mM increase of glucose concentration: (a) two adsorption cycles of GOx; (b) four adsorption cycles of GOx. |
An amperometric glucose sensor was fabricated by coating a phenylated graphene-polystyrene composite alternately with PDDA and GOx layers, where the graphene-polystyrene composite was deposited on polyester films. More than ten layers of GOx could be immobilized via electrostatic interaction by using a layer-by-layer adsorption method, where PDDA was the counter-ionic polymer. The adsorbed amount on the film was measured using QCM techniques, and the films were used as the working electrode for the glucose sensor. A precise response was obtained using the four adsorption cycles of GOx on the film.
- 1. Mohan, V. B.; Lau, K.; Hui, D.; Bhattacharyya, D. Graphene-based Materials and Their Composites: A Review on Production, Applications and Product Limitations. Composites Part B 2018, 142, 200-220.
-

- 2. Geim, A. K.; Novoselov, K. S. The Rise of Graphene. Nat. Mater. 2007, 6, 183-191.
-

- 3. Kuilla, T.; Bahdra, S.; Yao, D.; Kim, N. H.; Bose, S.; Lee, J. H. Recent Advances in Graphene Based Polymer Composites. Prog. Polym. Sci. 2010, 35, 1350-1375.
-

- 4. Gao, Y. Graphene and Polymer Composites for Supercapacitor Applications: a Review. Nanoscale Res. Lett. 2017, 12, 387.
-

- 5. Keyte, J.; Pancholi, K.; Njuguna, J. Recent Developments in Graphene Oxide/Epoxy Carbon Fiber-Reinforced Composites. Front. Meter. 2019, 6, 224.
-

- 6. Liu, N.; Luo, F.; Wu, H.; Liu, Y.; Zhang, C.; Chen, J. One-Step Ionic-Liquid-Assisted Electrochemical Synthesis of Ionic-Liquid-Functionalized Graphene Sheets Directly from Graphite. Adv. Funct. Mater. 2008, 18, 1518-1525.
-

- 7. Pinto, A. M.; Magalhaes, F. D. Graphene-Polymer Composites. Polymers 2021, 13, 685.
-

- 8. Marsden, A. J.; Papageorgiou, D. G.; Valles, C.; Liscio, A.; Palermo, V.; Bissett, M. A.; Young, R. J.; Kinloch, I. A. Electrical Percolation in Graphene-Polymer Composites. 2D Mater. 2018, 5, 32003.
-

- 9. Dideikin, A. T.; Vul, A. Y. Graphene Oxide and Derivatives: The Place in Graphene Family. Front. Phys. 2019, 6, 149.
-

- 10. Eissa, S.; Ndiaye, J.; Brisebois, P.; Izquierdo, R.; Tavares, A. C.; Siaj, M. Probing the Influence of Graphene Oxide Sheets Size on the Performance of Label-Free Electrochemical Biosensors. Sci. Rep. 2020, 10, 13612.
-

- 11. Senis, E. C.; Golosnoy, I. O.; Andritsch, T.; Dulieu-Barton, J. M.; Thomsen, O. T. The Influence of Graphene Oxide Filler on the Electrical and Thermal Properties of Unidirectional Carbon Fiber/Epoxy Laminates: Effect of Out-of-Plane Alignment of the Graphene Oxide Nanoparticles. Polym. Composite 2020, 41, 3510-3520.
-

- 12. Kim, J.; Campbell, A. S.; Esteban-Fernandez de Avila, B.; Wang, J. Wearable Biosensors for Healthcare Monitoring. Nat. Biotechnol. 2019, 37, 389-406.
-

- 13. Zhang, S.; Zeng, J.; Wang, C.; Feng, L.; Song, Z.; Zhao, W.; Wang, Q.; Liu, C. The Application of Wearable Glucose Sensors in Point-of-Care Testing. Front. Bioeng. Biotechnol. 2021, 9, 774210.
-

- 14. Liu, L.; Canoura, J.; Alkhamis, O.; Xiao, Y. Immobilization Strategies for Enhancing Sensitivity of Electrochemical Aptamer-Based Sensors. ACS Appl. Mater. Interfaces 2021, 13, 9491-9499.
-

- 15. Shende, P.; Patil, A.; Prabhakar, B. Layer-by-Layer Technique for Enhancing Physicochemical Properties of Actives. J. Drug Deliv. Sci. Tec. 2020, 56A, 101519.
-

- 16. Park, S.; Han, U.; Choi, D.; Hong, J. Layer-by-Layer Assembled Polymeric Thin Films as Prospective Drug Delivery Carriers: Design and Applications. Biomater. Res. 2018, 22, 29.
-

- 17. Lengert, E. V.; Koltsov, S. I.; Li, J.; Ermakov, A. V.; Parakhonskiy, B. V.; Skorb, E. V.; Skirtach, A. G. Nanoparticles in Polyelectrolyte Multilayer Layer-by-Layer (LbL) Films and Capsules-Key Enabling Components of Hybrid Coatings. Coatings 2020, 10, 1131.
-

- 18. Malucelli, G. Sol-Gel and Layer-by-Layer Coatings for Flame-Retardant Cotton Fabrics: Recent Advances. Coatings 2020, 10, 333.
-

- 19. Li, J.; Tan, S. N.; Ge, H. Silica Sol-Gel Immobilized Amperometric Biosensor for Hydrogen Peroxide. Anal. Chim. Acta 1996, 335, 137-145.
-

- 20. Lvov, Y.; Ariga, K.; Ichinose, I.; Kunitake, T. Assembly of Multicomponent Protein Films by Means of Electrostatic Layer-by-Layer Adsorption. J. Am. Chem. Soc. 1995, 117, 6117-6123.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2022 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2022; 46(5): 640-645
Published online Sep 25, 2022
- 10.7317/pk.2022.46.5.640
- Received on May 11, 2022
- Revised on Jul 18, 2022
- Accepted on Aug 2, 2022
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Conflict of Interest
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Min Jae Shin
-
Department of Cosmetics and Biotechnology, Semyung University, Jecheon 27136, Korea
- E-mail: newminj@gmail.com
- ORCID:
0000-0003-2068-6719










 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.