- Double-Layer Wound Dressing Consisting of an Upper Layer of Robust Polyurethane/Polycaprolactone and a Lower Layer of Biodegradable Polycaprolactone/Gelatin/Cellulose
Department of Biomedical Engineering, Daelim University, Anyang 13916, Korea
- 기계적 물성이 우수한 폴리우레탄/폴리카프로락톤 상층과 생분해성 폴리카프로락톤/젤라틴/셀룰로오스 하층으로 구성된 이중층 상처드레싱
대림대학교 보건의료공학과
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
Becausesingle-layer wound dressings cannot meet all clinical needs due to the complexity of the skin, a bilayer scaffold was synthesized by electrospinning a polyurethane/poly(caprolactone) (PU/PCL) top layer onto a spun PCL/gelatin/cellulose fiber (P/g/CF) sublayer using a common solvent to evaluate the feasibility of the scaffolds as a wound dressing. When the PU/PCL top layer was spun onto a P/g/CF sublayer, the tensile strength of the bilayer scaffold increased from 4.29 MPa to 6.34 MPa by adding a PU/PCL layer. Although the highest moisture vapor transmission rate (MVTR) and water uptake capacity (WUC) values were observed for PU/PCL and P/g/CF scaffolds, respectively, the appropriate MVTR and WUC values make all scaffolds applicable for wound dressings. Bilayer scaffold have potential applications in wound dressings due to their mechanical properties, MVTR, WUC, porosity, cell viability and proliferation.
단일층의 상처드레싱은 피부의 복잡성으로 인해 모든 임상 요구를 충족시킬 수 없다. 그렇기 때문에 이중층 스캐폴드의 상처드레싱이 사용된다. 전기방사를 이용하여 제조한 이중층 스캐폴등 상처드레싱은 하층에 폴리카프로락톤/젤라틴/셀루로오스(P/g/CF)를, 상층에 폴리우레탄/폴리카프로락톤(PU/PCL)으로 구성된다. P/g/CF에 PU/PCL을 전기방사함으로 스캐폴드의 기계적 강도는 4.29 MPa에서 6.34 MPa로 증가하였다. PU/PCL과 P/g/CF 단일지지체에서 최적의 투습도와 수분흡수도가 각각 관찰되었지만, 모든 지지체들은 상처드레싱에 적합하였다. 이중층 스캐폴드는 기계적 특성, 투습도, 수분흡수도, 세포생존률 및 증식이 상처 드레싱으로 사용하기에 적합하였다.
A bilayer scaffold was synthesized by electrospinning a polyurethane/poly(caprolactone) (PCL) top layer onto a spun PCL/gelatin/cellulose fiber sublayer to evaluate the feasibility of the scaffolds as a wound dressing. Bilayer scaffolds were determined to have potential applications in wound dressings due to their mechanical properties (6.34 MPa), moisture vapor transmission rate (1495 g/10 cm2·24h), water uptake capacity (203%), porosity (76%), cell viability (113%) and proliferation.
Keywords: wound dressing, bilayer scaffold, electrospinning, polyurethane, polycaprolactone.
This work was supported by a grant (code # 2022-022) from Gyeonggi-do research-centered R&D support project funded by Gyeonggi Province.
The authors declare that there is no conflict of interest.
Wound dressings should protect the skin from external contaminants and facilitate the healing process.1-8 Biopolymer membranes composed of synthetic and natural polymers play an important role in wound dressings due to their porosity, strength, moisture vapor transmission rate (MVTR), water uptake capacity (WUC), biocompatibility, and adequate biodegradability, but single-layer wound dressings cannot meet all clinical needs due to the complexity of the skin.4,6-10 Two-layer double membranes with different properties are emerging as the material of choice for wound dressings.7 A denser upper layer can protect the wound from infection and mechanical stress. In addition, this layer prevents dehydration of the wound and provides a moist environment for the wound site. The extracellular matrix (ECM)-like sublayer makes direct contact with the wound site to promote cell adhesion and proliferation.
Biocompatible natural polymers like gelatin exhibit rapid degradation. Poly(e-caprolactone) (PCL) synthetic polymer is a polyester characterized by non-toxic nature, slow degradation rate and high elasticity. The disadvantages of gelatin, such as rapid degradation and poor mechanical properties, can be overcome by proper alloying with synthetic polymer.4-6 Nanofibrous PCL/gelatin (P/g) scaffolds with specific proportions of gelatin and PCL exhibit appropriate mechanical properties and biological functions similar to natural tissues in cell attachment and proliferation.4,5 P/g composite scaffolds can be prepared using an inexpensive and less toxic acetic acid and ethyl acetate common solvent system. A 7/3 P/g scaffold loaded with 2 wt% cellulose fiber (CF) at 18% polymer concentration was determined to be an effective blend system for wound dressing due to its mechanical strength (4.8±0.8 MPa), MVTR (2450 g/10 cm2·24 h), WUC (716%), cell viability (114%) and proliferation.6 Our previous study revealed that the 10 wt% polyurethane/PCL (90/10, PU/PCL) scaffold showed a tensile strength of 12.1±1.4 MPa.11,12 It has excellent mechanical properties, biocompatibility, and circulation of nourishment and wastes, so it is likely to be applied as an artificial blood vessel with a small-diameter and porous structure. In this study, PU/PCL and P/g/CF layers are used as the top and bottom layers of wound dressing, respectively. The PU/PCL top layer is a dense structured membrane to protect the wound from external infection. The P/g/CF sublayer is a membrane with high ability to improve cell adhesion and proliferation. Mechanical properties, MVTR, WUC, porosity, and cytotoxicity of a bilayer wound dressing consisting of a dense PU/PCL top layer and a biodegradable P/g/CF sublayer are investigated.
Materials. PCL ((C6H10O2)n, Mw 80000), gelatin from porcine skin and CF, poly[4,4’-methylenebis(phenyl isocyanate)-alt-1,4-butanediol/di(propylene glycol)/polycaprolactone] (PU) were purchased from Sigma-Aldrich. Acetic acid (99.5%), ethyl acetate (99.5%), N,N dimethylformamide (DMF), tetrahydrofuran (THF), and NaOH (98%) were purchased from Samchun Pure Chemical Co., Ltd., Korea and used as they were received. A mixture of acetic acid, ethyl acetate, and distilled (DI) water was used as a common solvent for P/g/CF throughout the study.4-6 CFs immersed in ethanol were stirred for more than 3 h using ultrasound. Then, dried CF (2 wt%) was added to the 7/3 P/g solution and degassed for 10 min to obtain a homogeneous P/g/CF precursor solution.6 The preparation procedure for the PU/PCL precursor solution is described elsewhere.11,12
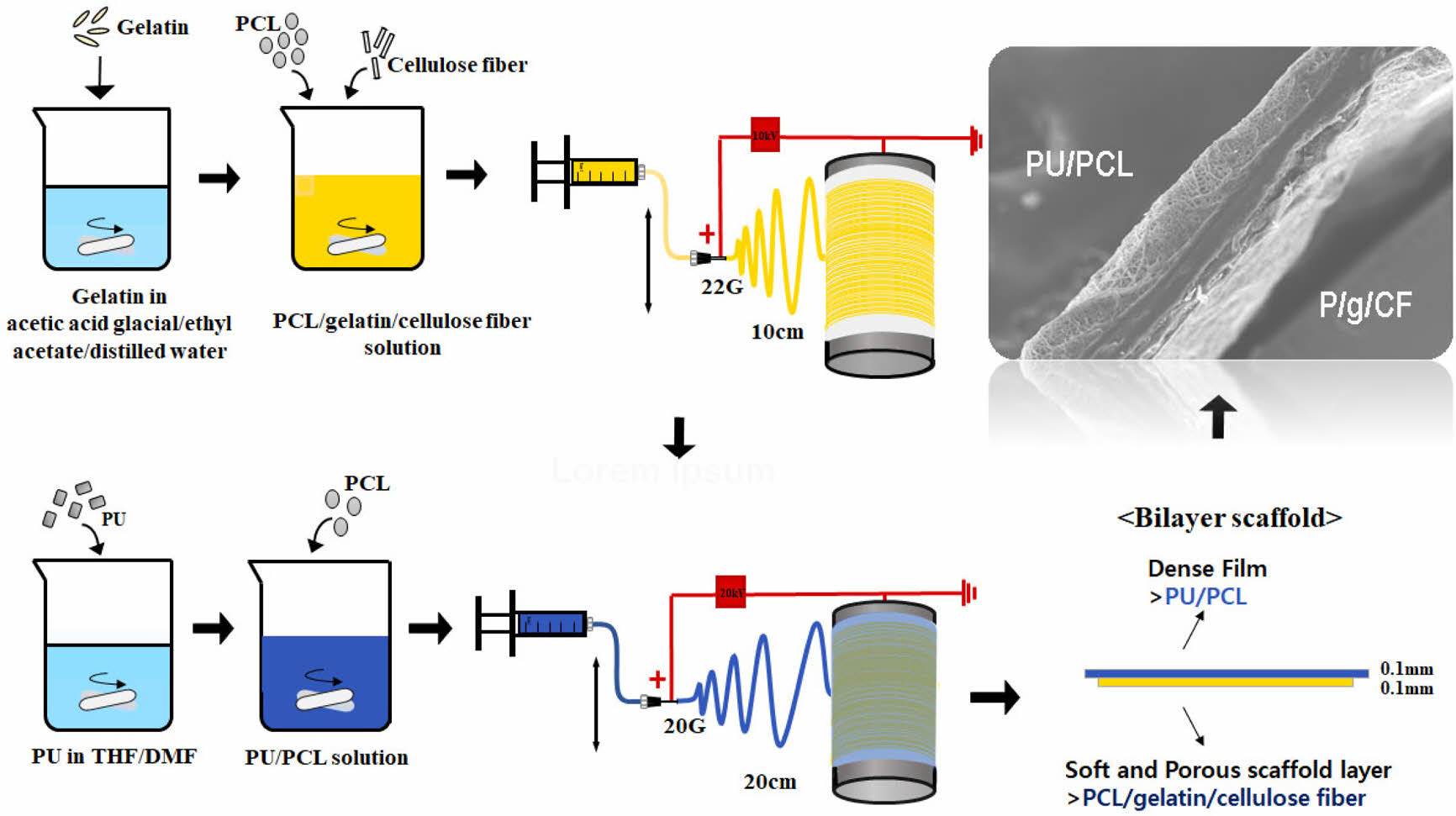
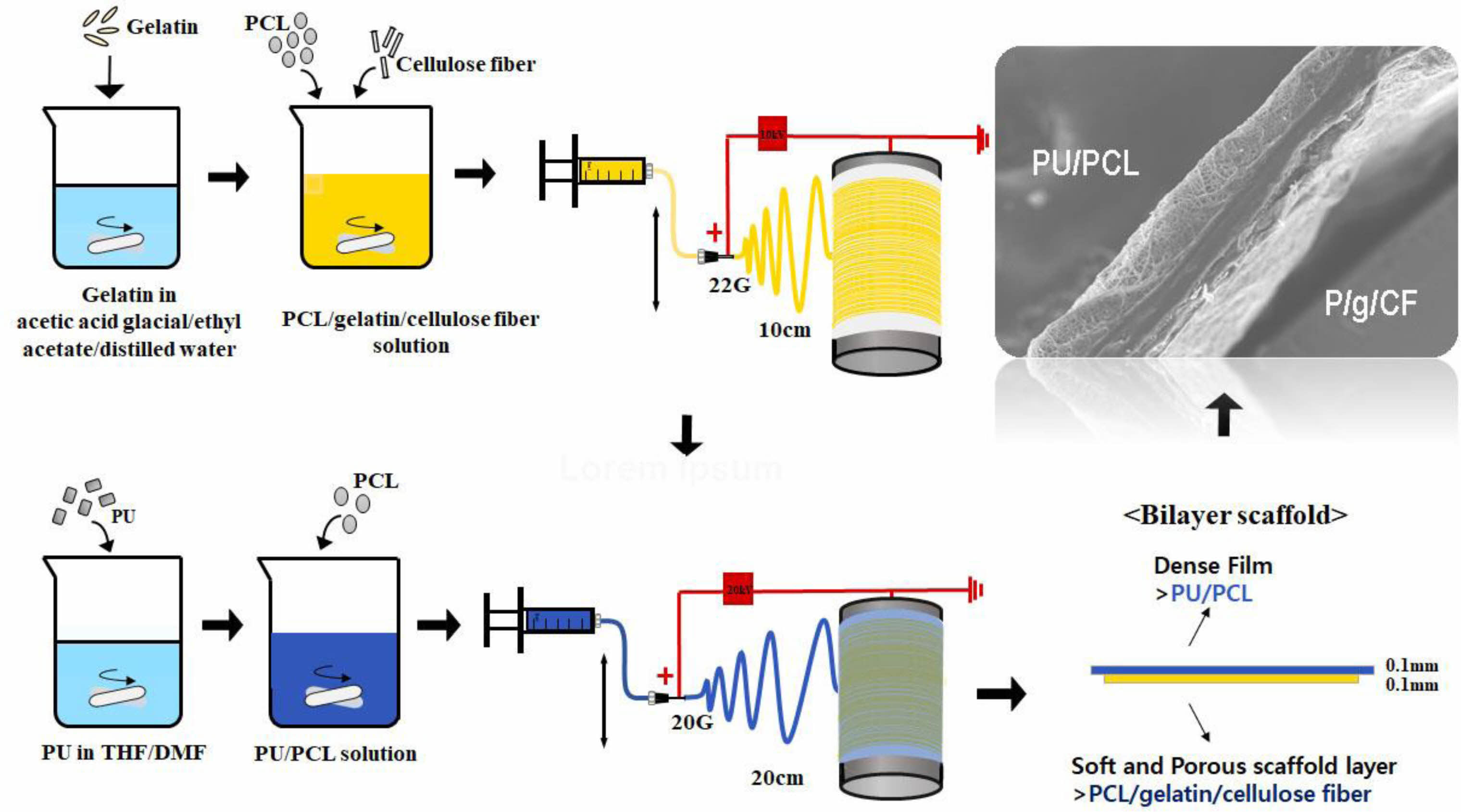
Electrospinning. The electrospinning apparatus consisted of a syringe pump (KDS-200, Stoelting Co., USA), a BD metal needle, a grounded collector, and a high-voltage power supply (ES30P-5W, Gamma High Voltage Research Inc., USA) equipped with current and voltage digital meters.4,6,11,12 The PU/PCL precursor solution was placed in a 5 mL BD luer-lock syringe attached to a syringe pump and fed to a 22 gauge metal needle at a flow rate of 1 mL/h. Membranes were collected at a voltage of 10 kV and a distance of 10 cm using a rotating metal drum with a diameter of 9 cm and a length of 20 cm. The rotational speed of the mandrel-type collector and the transverse speed of the needle were 250 rpm and 60 cm/min, respectively. The PU/PCL top layer was spun onto the as-spun P/g/CF layer for 6 h to obtain the 0.1 mm thickness layer, as shown in Figure 1. Detailed experimental procedure is described elsewhere.4,6,11
Scaffolds with a thickness of 0.2 mm were prepared. The scaffold was neutralized using 0.1 N NaOH solution and then washed three times with DI water. The as-prepared scaffolds were placed in a vacuum oven at 40 ℃ to eliminate remnant solvents. The scaffolds were then sterilized via exposure to ethylene oxide (EO) gas for 4 h with an EO sterilizer (HS-4313EO, Hanshin Medical, Korea).4,6,13,14
Characterization. Solution viscosity and electrical conductivity were measured at room temperature using a viscometer (DV 1M, Brookfield, USA) with spindle NO. SC4-31 at 20 rpm. The morphology of the scaffold was examined using a scanning electron microscope (SEM, S-3000H, Hitachi, Japan) and an optical stereomicroscope (SV-55, Sometech, Korea). Fiber diameters were then determined by using an optical microscope equipped with iSolution Lite image software.4,6,11,12 Fourier transform infrared spectroscopy (FTIR, Spectrum Two, PerkinElmer, UK) was used to analyze the chemical structure of the scaffolds.13,14 The mechanical properties of the scaffolds were investigated at room temperature using an Instron 5564 with a 1000 N force cell at a crosshead speed of 10 mm/min. Samples were prepared according to ASTM D-638 (type V) in the shape of a dumbbell. All experiments were performed in triplicate. The data were expressed as the mean ± standard deviation, and statistical significance was set at p < 0.05.4,6
MVTR, WUC, and Porosity of Scaffolds. MVTR of the scaffold is determined according to EN 13726-2:2002-Part 2.9,10 After fixing with a flat plate on one flange, 20 mL of distilled water was filled. A sample with a diameter of 55 mm was attached to the opposite side of the flange. The sample container was kept for 24 h at 37 ℃ and 20% humidity in a thermo-hygrostat. The liquid formed inside the wound layer dressing turns into a vapor state and is then transported to the atmosphere. The moisture vapor permeation helps wound healing by preventing wound infection. MVTR is then determined. As described elsewhere, the WUC and porosity of the scaffolds are also examined.4,6,8
Cytotoxicity and Cell Proliferation. The extract test method was performed on the scaffolds to evaluate the potential of cytotoxicity (L-929 mouse fibroblast cell (NCTC Clone 929, ATCC, USA)) on the base of the International Organization for Standardization (ISO 10993-5). The detailed experimental procedure is summarized elsewhere.4-6,13,14
|
Figure 1 Schematic diagram of electrospinning apparatus. |
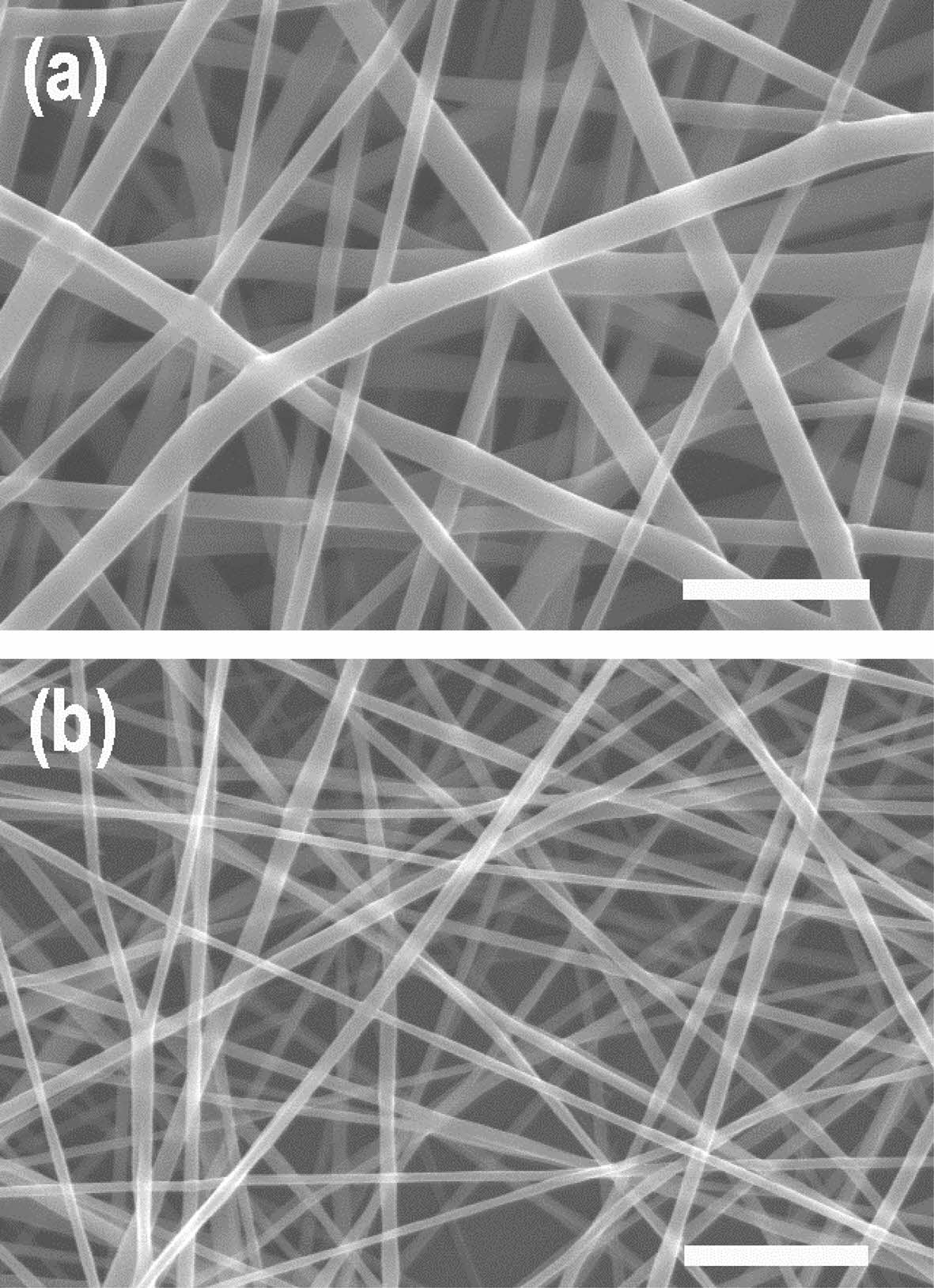
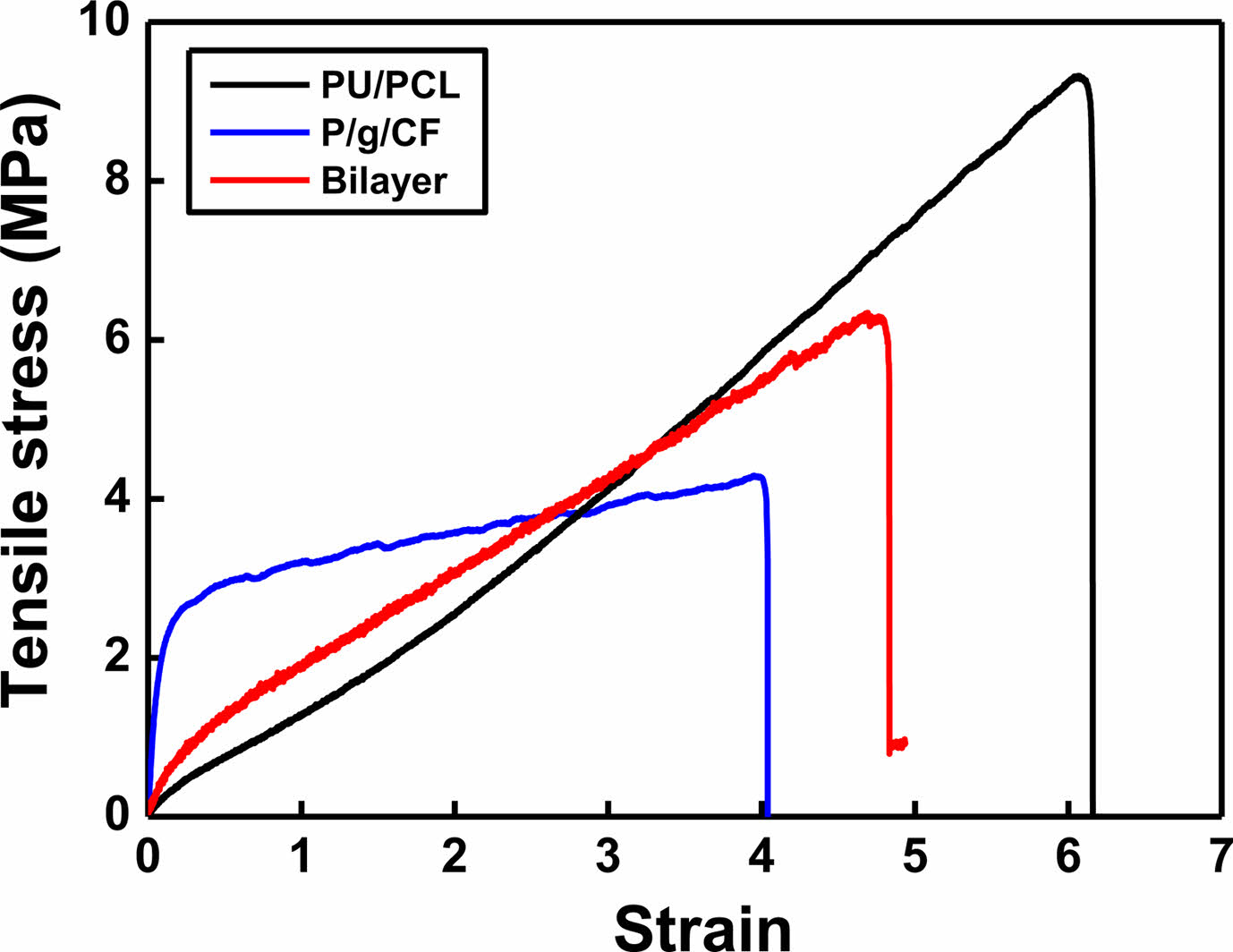
SEM images of the surface of the PU/PCL scaffold and the P/g/CF scaffolds loaded with 2 wt% CF are shown in Figure 2. Under the optimized conditions, the SEM images of the scaffolds exhibits a continuous and homogeneous fibrous morphology without bead formation. Fiber diameter was determined by measuring between 70 and 100 fibers.4,6 The average diameters of PU/PCL and P/g/CF scaffolds were 935 nm and 680 nm, respectively, in good agreement with the viscosities (1627 cP and 1025 cP). In our study, the porosity of PU/PCL, P/g/CF, and bilayer scaffolds was estimated to be 83%, 83%, and 76%, respectively, which are suitable for skin tissue engineering applications.4,7,15 It is reported that 60-90% porosity is ideal for scaffolds to facilitate fibroblast cell penetration and proliferation in the structure.13-15 The porous structure can also ensure sufficient gas and nutrient exchange to maintain homeostasis at the wound site. Tensile strength of PU/PCL, P/g/CF, and bilayer scaffolds were investigated, as illustrated in Figure 3. The PU/PCL scaffold exhibited the highest elongation at maximum load of 616%, and a tensile strength of 9.32 MPa. The point-bonding structure of the PU/PCL scaffold was suggested to be responsible for the load-bearing component.11,12 During deformation, most of the fibers were oriented in the axial direction, and the degree of point-bonding became more pronounced. The point-bonding structure of PU/PCL scaffolds can act as a load-bearing component under an applied force and as an obstacle to stress concentration.11 The P/g/CF scaffold showed a strength of 4.29 MPa at 400% elongation. The strength value (6.34 MPa at 484% elongation) of the bilayer scaffold was the median of the two single-layer scaffolds. The ideal tensile strengths for wound dressings and skin cell cultures range from 0.8 to 18 MPa.16,17 Experimental results show that the PU/PCL top layer are attributed to the mechanical properties of the bilayer scaffold, and the P/g/CF sublayer has no significant effect.8,11
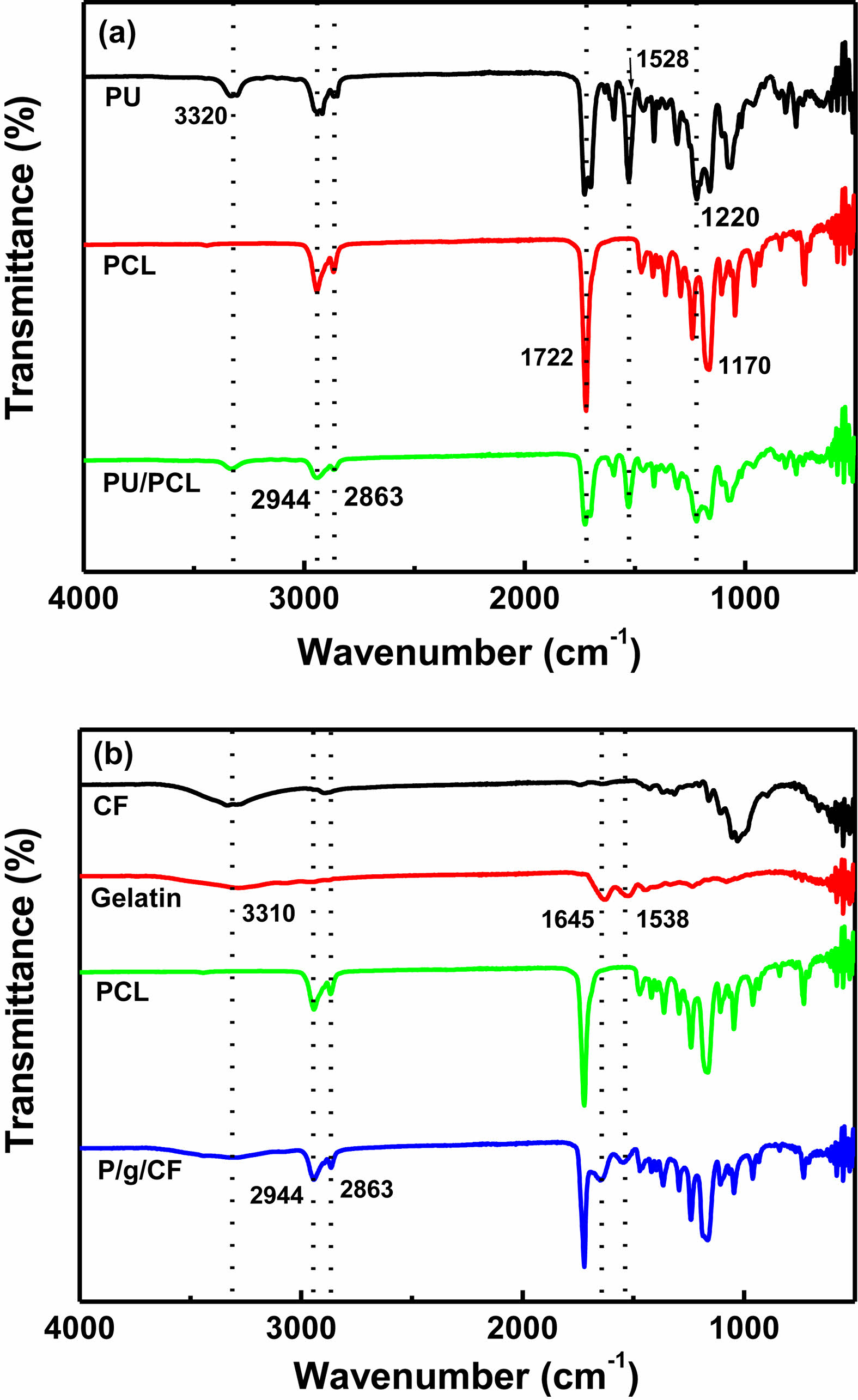
The FTIR spectra of PU/PCL, cellulose, gelatin, PCL, and P/g/CF scaffolds are shown in Figure 4. Characteristic -NH peaks of PU located at 3320, 1703, 1528, and 1220 cm-1 were observed (Figure 4(a)). Distinct peaks of the C-O and C-O-C bands in ester bond to PCL were observed at 1722 and 1170 cm-1, respectively. Asymmetric and symmetric peaks of -CH2 groups were observed in both PCL and PU at 2944 and 2863 cm-1, respectively, indicating the presence of a common functional group.16 However, no new bonds were identified in PU/PCL except for the peaks of PU and PCL, suggesting that there is no chemical reaction during blending of these polymers.11,16 Figure 4(b) shows that the amide (N-H stretching) peak of about 3300 cm-1 gelatin was weakened after loading CF on the P/g scaffolds.6 The PCL peaks present at 2945 cm-1 and 2865 cm-1, corresponding to the symmetrical and asymmetrical methylene groups, were slightly shifted to 2944 cm-1 and 2863 cm-1, respectively. The sharp intensities of amide bands (1645 cm-1 and 1538 cm-1) of P/g scaffold indicated good miscibility of the two polymers (PCL (-COO) and gelatin (-NH2)).4-6 The peak intensity and shape of gelatin with C=O stretching (amide I) at 1645 cm-1 and -NH bending (amide II) at 1538 cm-1 weakened and broadened after CF addition. The shift and broadening of these peaks could be an example of a presence interaction between the two polymers present in the blend and between cellulose and gelatin.5,6 The CF-loaded P/g scaffold favored hydrogen (H) bonds between cellulose (hydroxyl (-OH) and methylol (NH2·CH2OH) groups) and gelatin (carboxyl (-COOH) and amine groups (-NH2)) within the scaffold.5,6
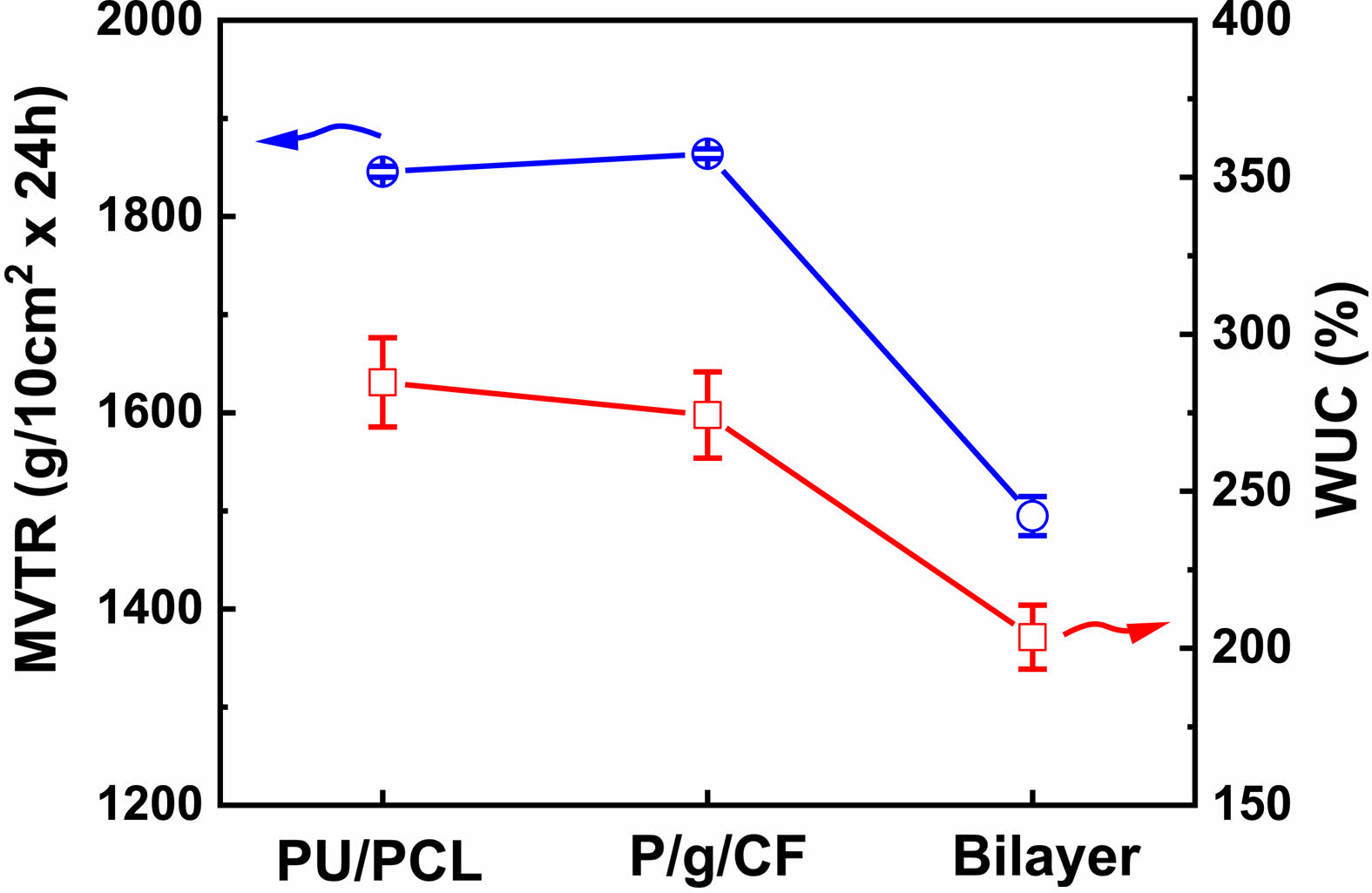
MVTR and WUC are important criteria for wound dressings requiring adequate healing.9,10 Low MVTR deposits produce exudates and delay the wound healing process, making the wound more susceptible to infection.9,10 On the other hand, high MVTR increases dehydration at the wound site, preventing the skin from being maintained for a long time in a proper moist environment, leading to scarring.4,6,9,10 It is recommended that the MVTR of wound dressing be in the range of ~500 to ~2000 g/10cm2·24h for suitable skin regeneration.4,9,10 MVTR and WUC values of PU/PCL, P/g/CF, and bilayer scaffolds are shown in Figure 5. The MVTR values of the PU/PCL, P/g/CF, and bilayer scaffolds are 1845 g/10 cm2·24 h, 1864 g/10 cm2·24 h, and 1495 g/10 cm2·24 h, respectively. All scaffolds prepared in this study can be considered as suitable biomaterials for infected skin, as the MVTR of wound dressing is likely to be in the range of ~500 to ~2000 g/10 cm2·24 h for proper skin regeneration.8 The WUC values (scaffold’s capacity to absorb wound exudate) of the PU/PCL, P/g/CF, and bilayer scaffolds are 285%, 274%, and 203%, respectively, as depicted in Figure 5. The lowest MVTR and WUC were observed in the bilayer scaffold. Although the highest MVTR and WUC values are observed for PU/PCL and P/g/CF scaffolds, respectively, any scaffold can be applied to wound dressings due to appropriate MVTR and WUC values.
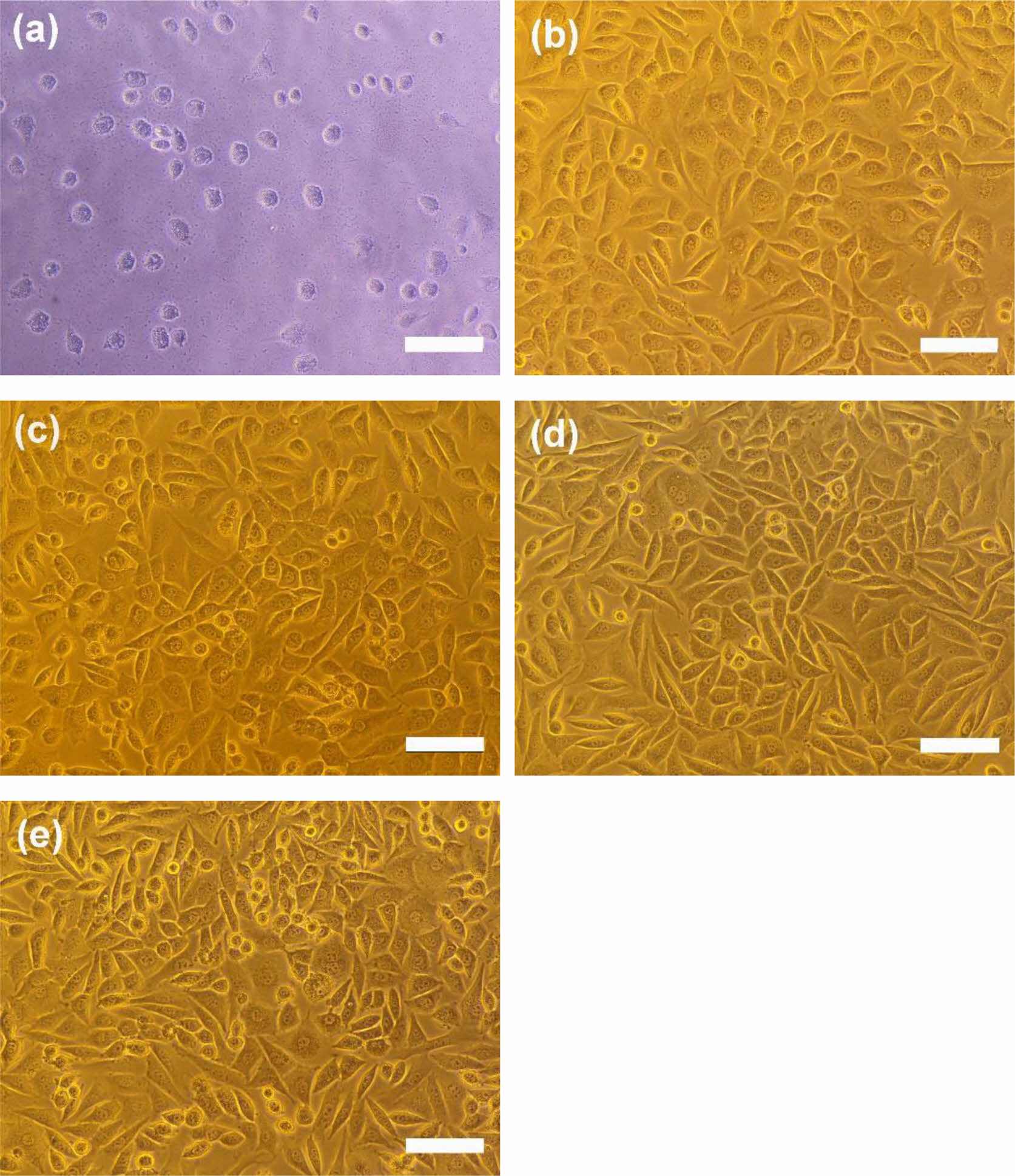
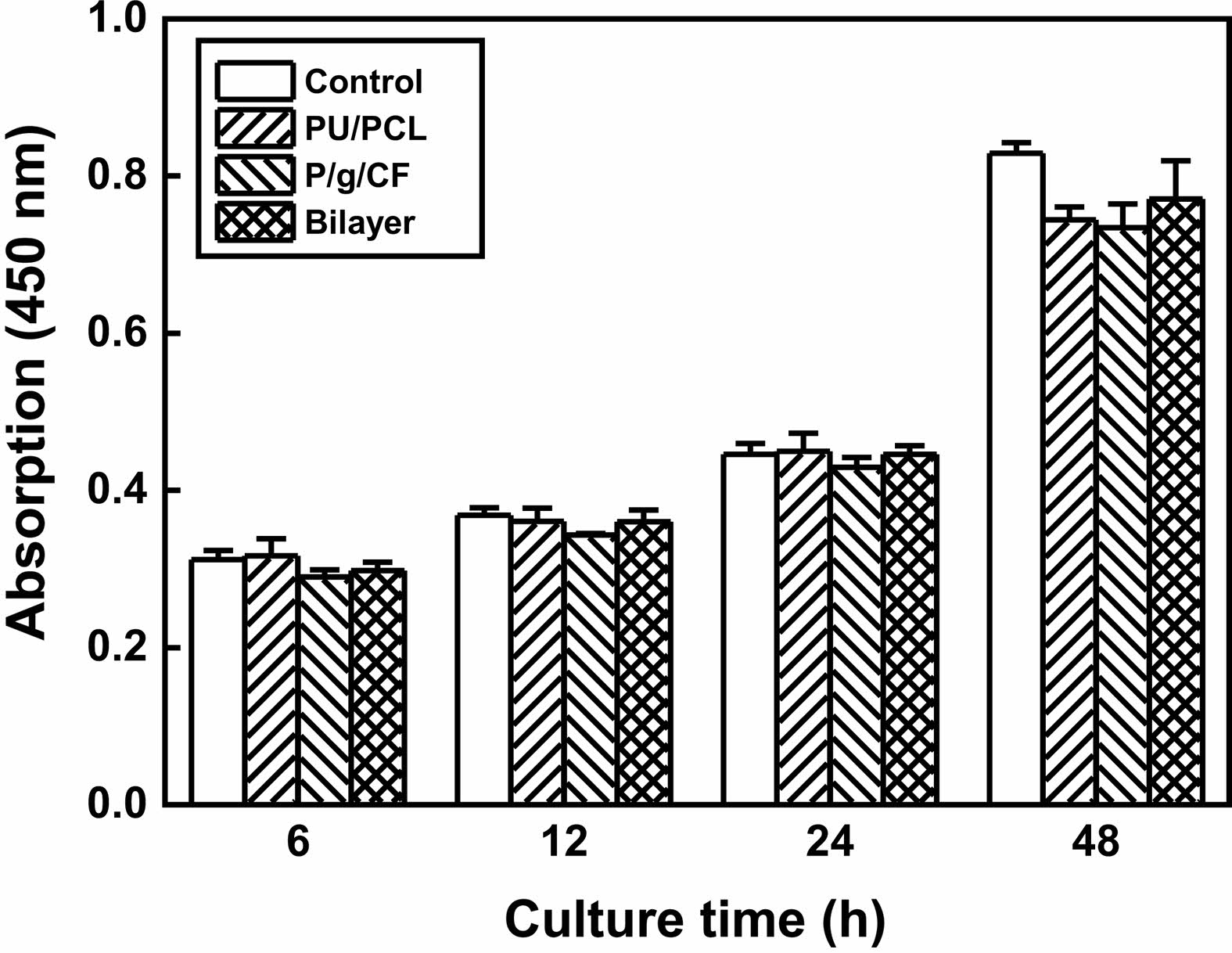
Cytotoxicity tests of PU/PCL, P/g/CF, and bilayer scaffolds determine whether the scaffolds have toxic effects on living cells. The test extracts showed no evidence of causing cell lysis or toxicity, as displayed in Figure 6. PU/PCL, P/g/CF, and bilayer scaffolds showed cell viability of 107%, 134%, and 113%, respectively, compared to the negative control, as measured at a wavelength of 415 nm by using the microplate absorbance spectrophotometer.4,6,11,12,18-21 As shown in Figure 7, the results of proliferation of L-929 cells on PU/PCL, P/g/CF, and bilayer scaffolds suggested that L-929 cells adhered well to the scaffolds and continued to proliferate over time.20 All scaffolds can be considered suitable as wound dressings because they are non-cytotoxic and have excellent cell adhesion and proliferation under the conditions of this study.8 A bilayer scaffold composed of a dense PU/PCL top layer and a biodegradable P/g/CF can be considered as the material of choice for wound dressings due to its tailored mechanical properties, physical properties, and biocompatibility.
|
Figure 2 SEM images of (a) PU/PCL; (b) P/g/CF scaffolds. Note that the scale bar is 5 µm. |
|
Figure 3 Mechanical properties of various scaffolds |
|
Figure 4 FTIR spectra of (a) PU/PCL; (b) P/g/CF scaffolds. |
|
Figure 5 MVTR and WUC of various scaffolds. |
|
Figure 6 Photographs of cell morphologies: (a) positive control; (b) negative control; (c) PU/PCL; (d) P/g/CF; (e) bilayer scaffolds. Scale bar is 100 µm. |
|
Figure 7 Proliferation of L-929 cells on negative control, PU/PCL, P/g/CF, and bilayer scaffolds over time. |
A 0.2 mm thick bilayer scaffold was synthesized by electrospinning a PU/PCL top layer on the top of the as-spun P/g/CF sublayer using a common solvent to evaluate the feasibility of the scaffolds as a wound dressing. When the PU/PCL layer was added to the P/g/CF layer, the tensile strength of the bilayer scaffold increased from 4.29 MPa to 6.34 MPa. The increase in strength was due to the presence of a PU/PCL layer with a point-bonding structure. Although the highest MVTR and WUC values are observed for PU/PCL and P/g/CF scaffolds, respectively, any scaffold can be applied to wound dressings due to appropriate MVTR and WUC values. The bilayer scaffold can be regarded as biomaterials for potential wound dressings due to its mechanical properties, MVTR, WUC, porosity, cell viability and proliferation.
- 1. Ghasemi-Mobarakeh, L.; Prabhakaran, M. P.; Morshed, M.; Nasr-Esfahani, M.; Ramakrishna, S. Electrospun Poly(e-caprolactone)/Gelatin Nanofibrous Scaffolds for Nerve Tissue Engineering. Biomater. 2008, 29, 4532-4539.
-

- 2. Gautam, S.; Dinda, A. K.; Mishra, N. C. Fabrication and Characterization of PCL/Gelatin Composite Nanofibrous Scaffold for Tissue Engineering Applications by Electrospinning Method. Mater. Sci. Eng. C 2013, 33, 1228-1235.
-

- 3. Binulai, N. S.; Natarajan, A.; Menon, D.; Bhaskaran, V. K.; Mony, U.; Nair, S. V. PCL-Gelatin Composite Nanofibers Electrospun Using Diluted Acetic Acid-Ethyl Acetate Solvent System for Stem Cell-based Bone Tissue Engineering. J. Biomater. Sci. 2014, 25, 325-340.
-

- 4. Song, Y.; Kim, B.; Yang, D. H.; Lee, D. Y. Poly(e-caprolactone)/Gelatin Scaffolds for Wound Dressing. Appl. Nanosci. 2022, 12, 3261-3270.
-

- 5. Goudarzi, Z. M.; Behzad, T.; Ghasemi-Mobarakeh, L.; Kharaziha, M.; Enayati, M. S. Structural and Mechanical Properties of Fibrous Poly(caprolactone)/gelatin Nanocomposite Incorporated with Cellulose Nanofibers. Polym. Bull. 2020, 77, 717-740.
-

- 6. Jeong, Y.; Lee, D. Y. Mechanical Properties and Biocompatibility of Electrospun Poly(e-caprolactone)/Gelatin Scaffolds Loaded with Cellulose Fibers. Polym. Korea 2022, 46, 837-842.
-

- 7. Eskandarinia, A.; Kefayat, A.; Agheb, M.; Rffienia, M.; Baghbadorani, M. A.; Navid, S.; Ebrahimpour, K.; Khodabakhshi, D.; Ghahremani, F. A Novel Bilayer Wound Dressing Composed of a Dense Polyurethane/Propolis Membrane and a Biodegradable Polycaprolactone/Gelatin Nanofibrous Scaffold. Sci. Rep. 2020, 10, 3063.
-

- 8. Salehi, M.; Niyakan, M.; Ehterami, A.; Haghi-Daredeh, S.; Nazarnezhad, S.; Abbaszadeh-Goudarzi, G.; Vaez, A.; Hashemi, S. F.; Rezaei, N.; Mousavi, S. R. Porous Electrospun Poly(e-caprolactone)/gelatin Nanofibrous Mat Containing Cinnamon for Wound Healing Application, in vitro and in vivo Study. Biomed. Eng. Lett. 2020, 10, 149-161.
-

- 9. Kuppan, P.; Sethuraman, S.; Krishnan, U. M. PCL and PCL-Gelatin Nanofibers as Esophageal Tissue Scaffolds: Optimization, Characterization and Cell-matrix Interactions. J. Biomed. Nanotechnol. 2013, 9, 1540-1555.
-

- 10. Chellamani, K.P.; Sundaramoorthy, P.; Suresham, T. Wound Dressing Made out of Polyvinyl Alcohol/Chitosan Nanomembranes. J. Acad. Indus. Res. 2012, 1, 342-347.
- 11. Oh, G.; Rho, J.; Lee, D. Y.; Lee, M.; Kim, Y. Synthesis and Characterization of Electrospun PU/PCL Hybrid Scaffolds. Macromol. Res. 2018, 26, 48-53.
-

- 12. Seol, B.; Shin, J.; Oh, G.; Lee, D. Y.; Lee, M. Characteristics of PU/PEG Hybrid Scaffolds Prepared by Electrospinning. J. Biomed. Eng. Res. 2017, 38, 248-255.
- 13. Shin, J.; Lee, D. Y.; Kim, B.; Yoon, J. I. Effect of Polyethylene Glycol Molecular Weight On Cell Growth Behavior of Polyvinyl Alcohol/Carboxymethyl Cellulose/Polyethylene Glycol Hydrogel. J. Appl. Polym. Sci. 2020, 137, 49568.
-

- 14. Shin, J.; Lee, D. Y.; Yoon, J. I. Effect of CMC Concentration on Cell Growth Behavior of PVA/CMC Hydrogel. Macromol. Res. 2020, 28, 813-819.
-

- 15. Sundaramurthi, D.; Krishnan, U. M.; Sethyraman, S. Electrospun Nanofibers as Scaffolds for Skin Tissue Engineering. Polym. Rev. 2014, 54, 348-376.
-

- 16. Kandi, R.; Pandey, P. M. Fabrication and Characterization of Customized Tubular Scaffolds for Tracheal Tissue Engineering by Using Solvent Based 3D Printing on Predefined Template. Rapid Prototyping J. 2021, 27, 421-428.
-

- 17. Gomes, S. R.; Rodrigues, G.; Martins, G. G.; Roberto, M. A.; Mafra, M.; Henriques, C. M. R.; Silva, J. C. In vitro and in vivo Evaluation of Electrospun Nanofibers of PCL, Chitosan and Gelatin: A Comparative Study. Mater. Sci. Eng. C 2015, 46, 348-358.
-

- 18. Jeong, H.; Rho, J.; Shin, J.; Lee, D. Y.; Hwang, T.; Kim, K. J. Mechanical Properties and Cytotoxicity of PLA/PCL Scaffolds. Biomed. Eng. Lett. 2018, 8, 267-271.
-

- 19. Kim, D.; Lee, M.; Lee, D. Y.; Han, J. Mechanical Properties, Phase Stability, and Biocompatibility of (Y,Nb)-TZP/Al2O3 Composite Abutments for Dental Implant. J. Biomed. Mater. Res. 2000, 53, 438-443.
-

- 20. Longhao, J.; Park, K.; Yoon, Y.; Kim, H. S.; Kim, H. Y.; Choi, J.W.; Lee, D. Y.; Chun, H. J.; Yang, D. H. Visible Light-cured Antibacterial Collagen Hydrogel Containing Water-Solubilized Triclosan for Improved Wound Healing. Mater. 2021, 14, 2270.
-

- 21. Ke, R.; Yi, W.; Tao, S.; Wen, Y.; Hongyu, Z. Electrospun PCL/gelatin Composite Nanofiber Structures for Effective Guided Bone Regeneration Membranes. Mater. Sci. Eng. C 2017, 78, 324-332.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2022 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2023; 47(2): 151-156
Published online Mar 25, 2023
- 10.7317/pk.2023.47.2.151
- Received on Oct 25, 2022
- Revised on Dec 7, 2022
- Accepted on Dec 28, 2022
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
- Conflict of Interest
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Deuk Yong Lee
-
Department of Biomedical Engineering, Daelim University, Anyang 13916, Korea
- E-mail: dylee@daelim.ac.kr
- ORCID:
0000-0003-1674-412X










 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.