- Changes in Glioblastoma Cell Characteristics by a Strong Anionic Poly(Cresolsulfonic Acid) and a Cationic Pyrvinium Complex
Department of Chemical Engineering, College of Engineering, Dankook University, Jukjeon-dong, Yongin-si, Gyeonggi-do 16890, Korea
- 강한 음이온성 폴리(크레졸술폰산)과 양이온성 피르비늄 복합체에 의한 교모세포종 세포 특성 변화
단국대학교 화학공학과
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
Basic cell studies on a strong anionic poly(cresolsulfonic acid) and a cationic pyrvinium have been conducted to develop therapeutic drugs for glioblastoma, the most malignant brain tumor. To determine the optimal concentration of these mixed drugs, experiments on the cell shape change and survival rate were conducted, and cell resistance was tested through the re-administration of drugs. Through immunochemical testing, the mechanism for cell morphological changes and inhibition of proliferation could be inferred, and when used in combination with temozolomide, the mixture showed a greater cytotoxic effect. Further research is needed on the mechanisms, cell reactions, and animal testing, with a possibility to develop it as a therapeutic drug.
가장 악성 뇌종양인 교모세포종 치료제를 개발하기 위해 강력한 음이온성 고분자인 폴리(크레졸술폰산)와 양이온성 피르비늄에 대한 기초 세포 연구가 진행됐다. 이러한 혼합 약물의 최적 농도를 결정하기 위해 세포 형태 변화와 생존율에 대한 실험을 수행하고, 약물의 재투여를 통해 세포 저항성을 실험하였다. 면역화학적 검사를 통해 세포 형태학적 변화와 증식 억제 메커니즘을 유추할 수 있었으며, 테모졸로마이드와 병용할 경우 혼합물이 더 큰 세포독성 효과를 보였다. 치료약으로 개발할 수 있는 가능성과 함께 메커니즘, 세포 반응 및 동물 실험에 대한 추가 연구가 필요하다.
Basic cell research using poly(cresolsulfonic acid) and pyrvinium was conducted to develop a treatment for glioblastoma, a malignant brain tumor, and significant results were confirmed.
Keywords: glioblastoma, pyrvinium, poly(cresolsulfonic acid), temozolomide, brain tumor.
The present research was conducted by the research fund of Dankook University in 2023
The authors declare that there is no conflict of interest.
Glioblastoma (GBM) is the most common malignant brain tumor, comprising of 15% of the total malignant brain tumors. The World Health Organization (WHO) has classified astrocytomas into four grades (I, II, III, and IV), among which invasive tumors, especially high-grade (grade IV) glioma, has a poor prognosis, with a median overall survival of only 11 months in the general GBM population and a five-year mortality rateofmore than 90%.1,2 Although such tumors rarely metastasize to distant tissues, they grow characteristically by infiltrating the surrounding brain tissues.3 The standard care therapy for GBM includes safe maximal surgical resection of the accessible tumor, followed by radiotherapy (RT) and chemotherapy with temozolomide (TMZ).4 However, despite these approaches, GBM remains incurable, and more than two-thirds of GBM patients die within two years of diagnosis.5 TMZ is the only chemotherapeutic drug licensed and used for GBM; however, the fact that GBM cells easily and quickly show resistance to TMZ limits its use. In addition, the clinical effect of TMZ provides only a modest two month increase in the median survival compared with the effects with no medication.6 Therefore, a new strategy is needed to improve the prognosis of patients with GBM. Molecular targeted therapies, such as bevacizumab, cetuximab, or nimotuzumab are the emerging treatments for GBM.7 Moreover, immunotherapies, such as dendritic cells, natural killer cells, and suicide gene therapy, have become the focus of current research.8-10 However, these drugs and therapies do not efficiently improve the survival rates and have the disadvantages of severe individual differences. According to recent studies, growing spheroids in an incubated cancer cell matrix can result in the enrichment of cancer stem cells (CSCs), which are believed to be major contributors to drug resistance and tumor recurrence.11-14 CD133 is a membrane-bound pentaspan glycoprotein first identified in neuroepithelial stem cells, and has been identified in various carcinomas, including CSCs.15,16 CD133 appears to be associated with the Wnt signaling pathway for cell proliferation17,18 and has been known to profoundly affect the survival of patients with cancer.19,20
As a cathodic materials, pyrvinium, an food and drug administration (FDA)-approved effective anthelmintic drug, inhibits the oxidative metabolism and glucose uptake in pinworms and possibly demonstrates anticancer activity by the inhibiting CD133 positive cancer stem cells through the suppression of Wnt/β-catenin signaling. Therefore, several studies for cancer cells that express CD133, such as ovarian cancer cells and GBM, are underway as a research target for pyrvinium.21-23
GBM is also called “octopus tumor” because it can extend its tendrils to normal neighboring parenchymal cells.24,25 In addition, unlike normal cells, cancer tissues grow and metastasize at a high speed by invading the surrounding normal tissues in a vine-like twisted form.
Cadherins are essential components of adherens junction complexes that mediate cell-cell adhesion and regulate cell motility. Epithelial-mesenchymal transition (EMT), principally involving an E-cadherin to N-cadherin shift, is linked to tumor invasion or metastasis and therapeutic resistance in various cancers.26 A growing body of evidence supports the hypothesis that EMT plays a crucial role in the invasive phenotype of gliomas. According to a recent study, E-cadherin is the predominant cadherin expressed in epithelial tissues; however, its expression is very limited in the normal brain. In contrast, E-cadherin expression has been confirmed to have a significant influence on the in vitro growth and invasion in glioma cell models.27,28
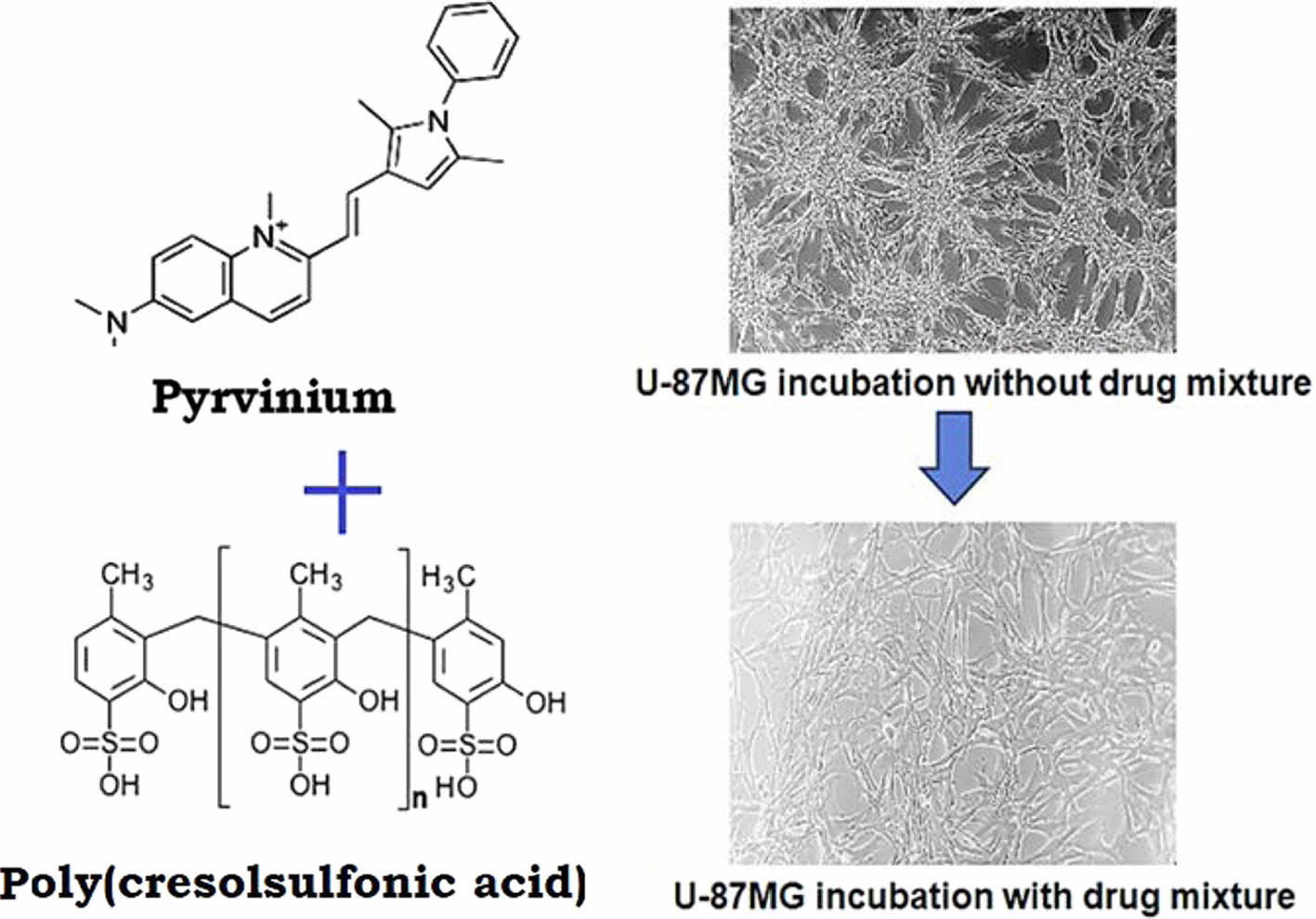
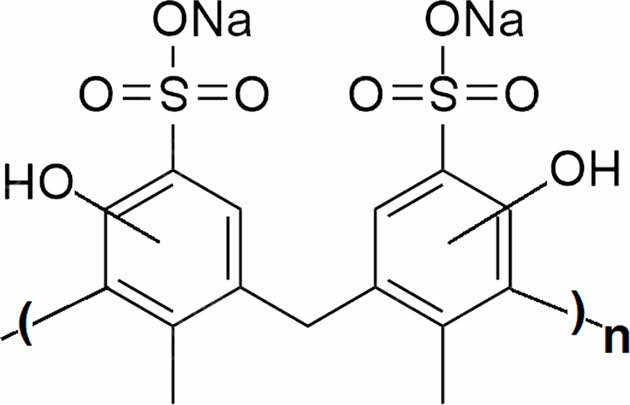
As an anionic polymer, poly(cresolsulfonic acid) is the polycondensation product of cresol sulfonic acid and phenol and belongs to the water-soluble polymer. It is sold under the name policyresulen on the market. Poly(cresolsulfonic acid), which has the same structure as in Figure 1, is a strong acidic polymer that mainly reacts to blood and exhibits hemostasis and wound healing effects.
It is usually used as a topical hemostatic and antiseptic agent for infectious and other lesions of the mucous membrane, such as gynecological infections, anal hemorrhoids, and oral ulcers.29 According to previously reported research results, the application of poly(cresolsulfonic acid) reported a significant reduction in the expression of E-cadherin in animals, which indicated decreased cellular attachments with a reduction in the E-cadherin expression level.30,31
Therefore, as a basic cell study for drug search, the objectives of this study were as follows: (a) to determine the ideal combination of cathodic pyrvinium and as an anionic polymer, poly(cresolsulfonic acid) for anticancer activity, (b) to observe the change in the spherical proliferation of incubated cells, and (c) to measure the cell morphological changes caused by the mixture.
|
Figure 1 Chemical structure of shellac poly(cresolsulfonic acid). |
Materials. In this study, poly(cresolsulfonic acid) (Policresulen, Takeda Pharmaceutical Company, Albothyl, TK. JP) at a concentration of 50 wt% was used to inhibit aggressive cell infiltration. Pyrvinium pamoate salt hydrate was used as an inhibitor of cancer stem cell proliferation, and the WST-8 assay kit, TMZ, dimethylsulfoxide (DMSO), and cell culture reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA). All the other chemicals used were of analytical grade. Ultrapure water was obtained from a Milli-Q water system and used to prepare the aqueous solutions.
Determination of Medium Conditions for Cell Culture. Two types of cells were used: human GBM cells as cancer cells and fibroblasts as normal cells. To confirm the conditions of cell culture by the fetal bovine serum (FBS) contents for drug effect, a culture medium [Dulbecco's modified Eagle’s medium (DMEM) supplemented with 2 mM L-glutamine] containing 0, 1, 5, and 10% FBS was prepared. Each of the NIH-3T3 cells (ATCC-L929, Manassas, VA, USA) and U-87MG cells (30014, Seoul, KR) were added (2×104 cells per well) to the 6 well plate. After 1 h, only the medium was removed, 2 mL of the previously prepared medium containing 0, 1, 5, and 10% FBS was added, and the proliferation was quantified after 4, 24, 48, 72, and 96 h using a WST-8 assay.
Cell Response Test Using Pyrvinium. Based on the previous results, a cell culture medium containing 5% FBS was prepared and used. The NIH-3T3 and U-87GM cells were added directly to each plate in 200 µL of media suspension. The cell density was set at 2×104 cells per well. After 1 h, 1800 µL of medium was added to each well.
Pyrvinium was dissolved in DMSO in a concentration of 1 mM and subsequently added to 2 mL of the medium solution in which the cells were cultured to attain final concentrations of 0, 50, 75, 100, 125, 150, 175, 200, 250, and 300 nM, respectively. The proliferation was quantified after 12, 24, 48, 72, and 96 h using a WST-8 assay.
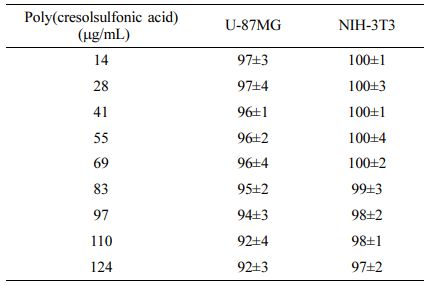
Cell Response Test Using Poly(Cresolsulfonic Acid). The same procedure was performed for pyrvinium, and the final concentrations of poly(cresolsulfonic acid) dissolved in PBS were added to the medium to obtain final concentrations of 0, 14, 28, 41, 55, 69, 83, 97, 110, and 124 µg/mL. The proliferation was quantified after 12, 24, 48, 72, and 96 h using the WST-8 assay.
Cell Response Test Using Pyrvinium and Poly(Cresol- sulfonic Acid) Mixture. This experiment was conducted under the same conditions as in the previous cell experiments. After preparing a 2 mL medium to obtain final concentrations of 50/28 µg/mL, 75/41 µg/mL, 100/55 µg/mL, 125/69 µg/mL, 150/83 µg/mL, 175/97 µg/mL, 200/110 µg/mL, and 250 nM/124 µg/mL as poly(cresolsulfonic acid)/pyrvinium mixtures, the effects on the proliferation of NIH-3T3 and U-87MG cells were measured and quantified every 12, 24, 48, 72, and 96 h.
Cell Responses Upon Drug Removal and Re-drugging. The drug-mixed medium was completely removed from the U-87MG cells that were cultured for 72 h in a mixture of 110 µg/mL poly(cresolsulfonic acid) and 200 nM pyrvinium. After washing with PBS three times, 2 mL of fresh medium without drugs was added, observed for 72 h, and the survival rate was quantified. Subsequently, the medium was completely removed and replaced with a medium containing poly(cresolsulfonic acid) (110 µg/mL) and pyrvinium (200 nM), and was observed and quantified for 72 h.
Immunocytochemistry. The U-87MG cells in chamber slides were fixed with 4% formalin and incubated with a blocking solution containing 1% bovine serum albumin in PBS. The antibodies used in this study were as follows: anti-rabbit CD133 (Cat # PA5-38014, Thermo Fisher), anti-mouse ACTC1(Cat # MAB9308, R&D Systems), Alexa 488 goat-anti-rabbit secondary antibody (Cat # A-11034, Invitrogen), and Alexa 594 goat anti-mouse secondary antibody (Cat # A-11032, Invitrogen). The U-87MG cells were incubated with primary antibody for 2 h and subsequently with secondary antibody for 1 h. Further, the cells were stained with 4,6-diamidino-2-phenylindole (DAPI)-containing agent (Vector, Burlingame, Calif., USA), and were examined using a fluorescence microscope.
Cell Responses in the Mixing of Temozolomide, Pyrvinium, and Poly(Cresolsulfonic Acid). TMZ was dissolved in DMSO to a concentration of 1 mM and subsequently added to 2 mL of the medium solution in which the U-87MG cells were cultured to make final concentrations of 0, 100, 250, 500, 750, and 1000 µM, respectively. In addition, after 72 h, the cell responses were compared with that of TMZ alone and TMZ mixtures [with poly(cresolsulfonic acid) 110 µg/mL and pyrvinium 200 nM] using a WST-8.
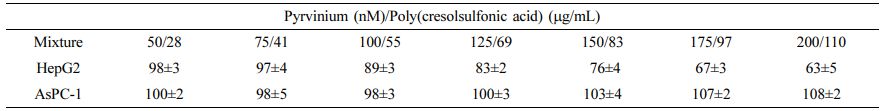
Drug Reactions Using Liver and Pancreatic Cancer Cells. The process was the same way as reported in Section 2.5; human HepG2 (hepatoblastoma, 88065, Seoul, KR) and human AsPC-1 (adenocarcinoma, 21682, Seoul, KR) cells were used. After 72 h, the cell state was observed and quantified.
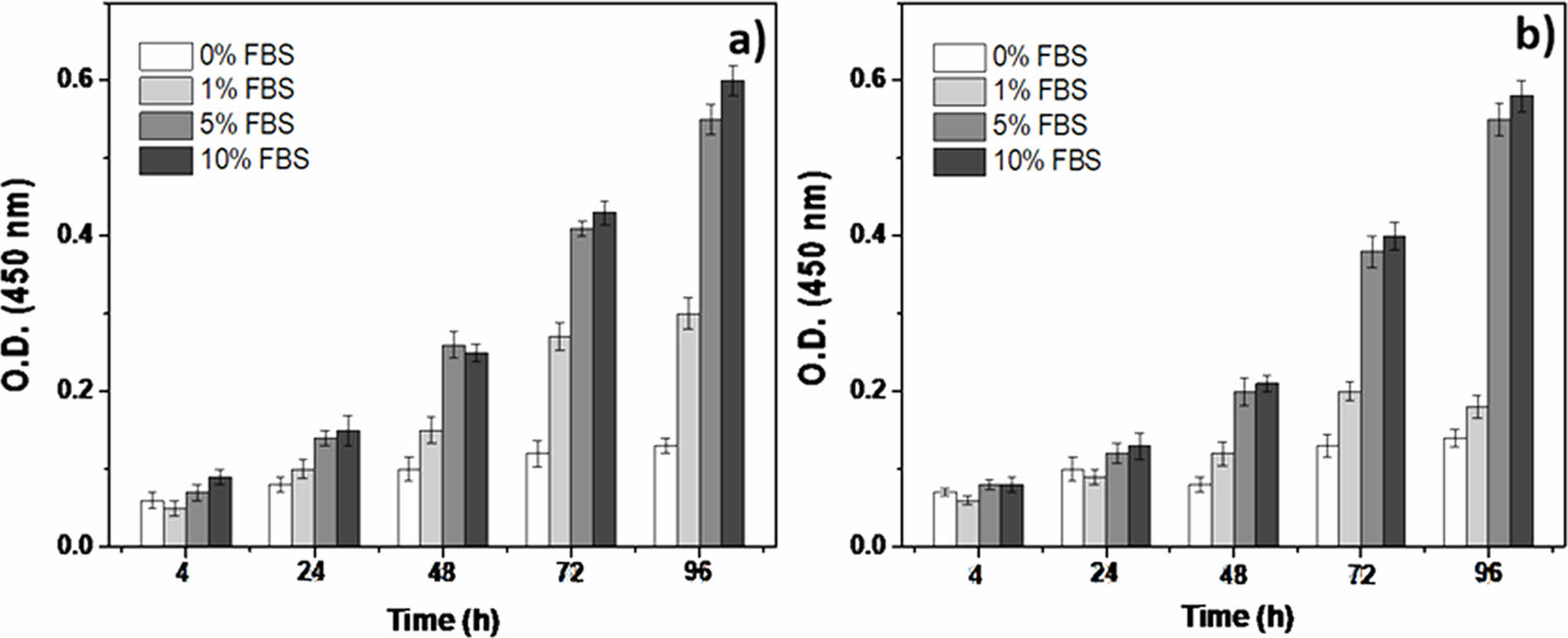
Determination of Cell Medium Composition. Figure 2 illustrates the results of culturing cells in a medium containing 0% to 10% FBS. The purpose of this study was to observe the effect of drugs on cell proliferation and changes in the shape compared to the findings from cell death studies that used anticancer drugs. Therefore, in the case of 0% FBS, no apparent proliferation of cells was observed in either cell group during starvation. However, when 1% FBS was applied, there was a weak cell proliferation effect in U-87MG cells; however, visible cell growth was weak in NIH-3T3 cells. Therefore, based on this experiment, the minimum amount of FBS that allowed smooth cell proliferation was set at 5%, and a medium with 5% FBS was used for subsequent cell culture.
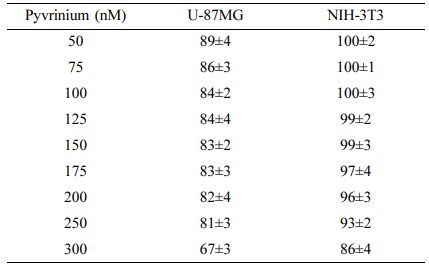
A Study on the Cell Response Using Pyrvinium. The results of measuring the survival rate of cells according to the culture time at various concentrations of pyrvinium are summarized in Table 1 as the culture results after 96 h for convenience. The cell culture results according to the concentration of pyrvinium showed a similar tendency in both the cell groups. At concentrations of up to 250 nM, both cell groups demonstrated cytotoxicity at approximately 10% and relatively high cytotoxicity at 300 nM. As normal and cancer cells showed similar tendencies, the following experiment was conducted in a concentration range below this concentration due to a non-specific cytotoxic reaction at 300 nM.
A Study on the Cell Response Using Poly(Cresolsulfonic Acid). The results of measuring the survival rate of cells according to the culture time at various concentrations of poly(cresolsulfonic acid) are summarized in Table 2 as the culture results after 96 h for convenience. The water-soluble poly(cresolsulfonic acid) used in this study was mixed with a cell culture medium as an acidic material and the pH change was measured. It was confirmed that it did not affect the pH change of the existing medium and an experiment was performed. Unlike pyrvinium, poly(cresolsulfonic acid) showed relatively low toxicity for both cell groups, and no specific response to cancer cells was observed in the single administration state.
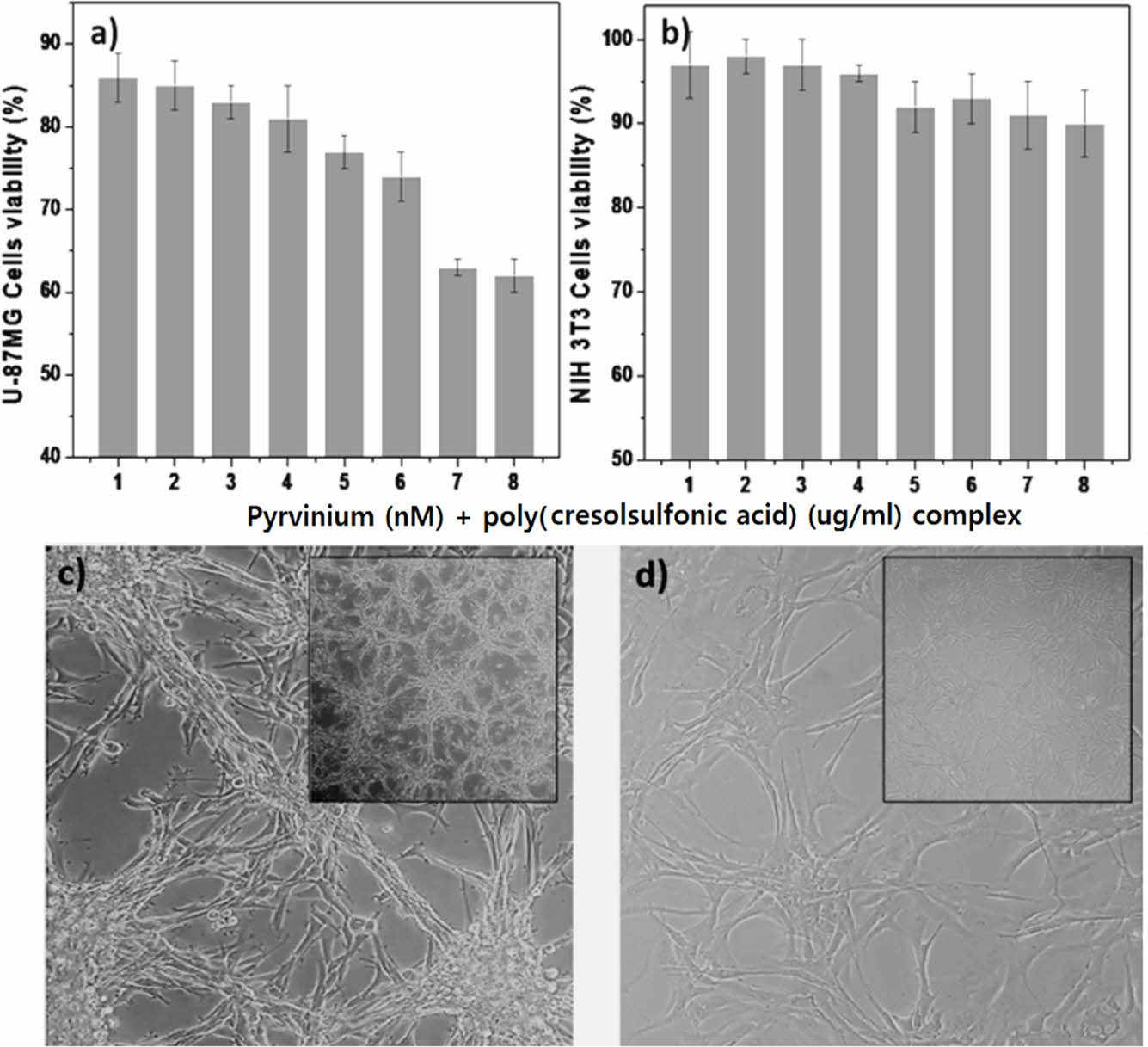
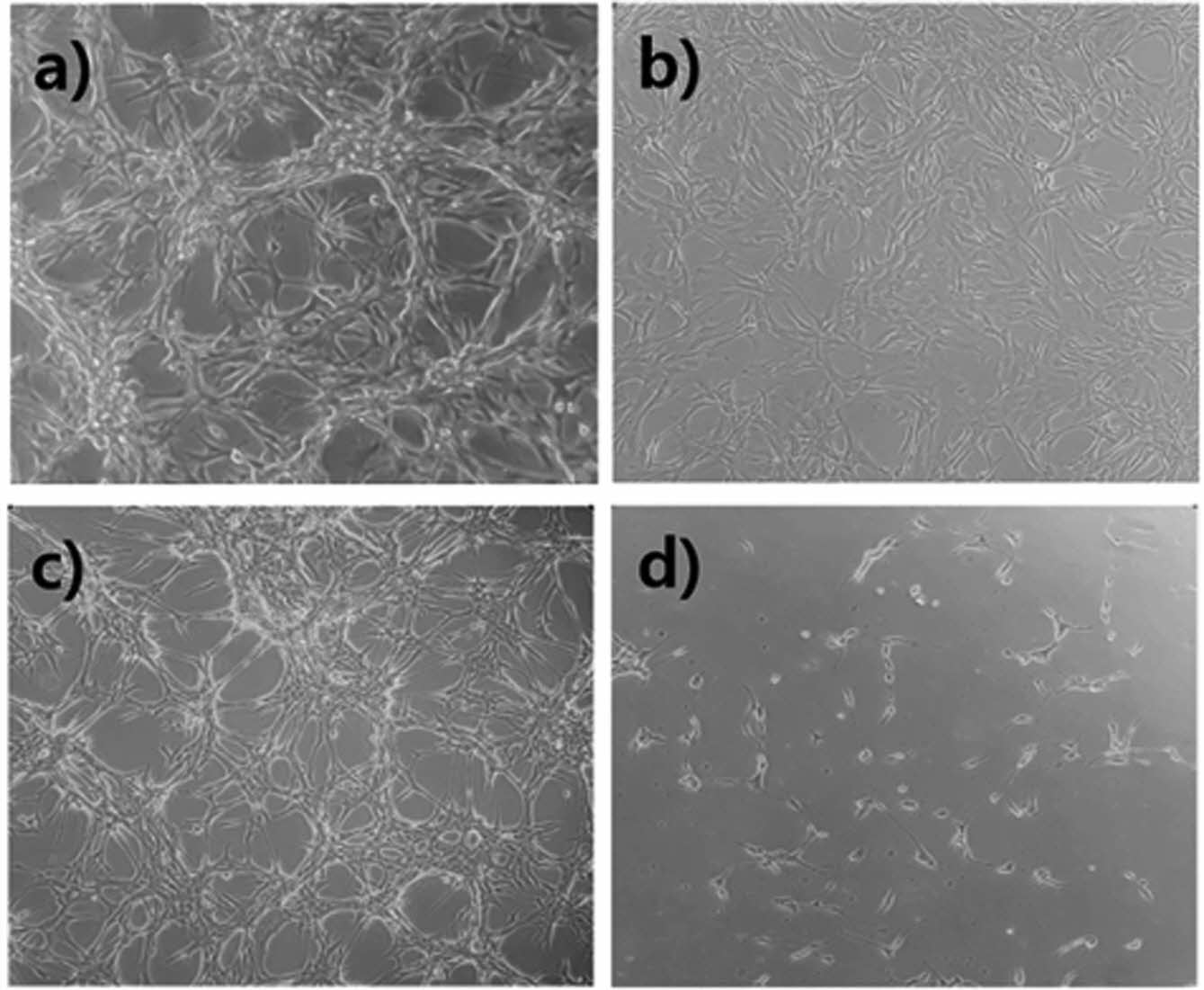
A Study on the Cell Response Using a Pyrvinium/Poly(Cresolsulfonic Acid) Mixture. Figure 3 shows the results of observing the response of cells while increasing the amount of pyrvinium and poly(cresolsulfonic acid). As shown in Figure 3(a), the degree of cell proliferation was slightly inhibited as the amount of the mixture increased in U-87MG cells, whereas no significant cytotoxicity was observed in NIH-3T3 cells (Figure 3(b)). Through this experiment, the mixture of pyrvinium and poly(cresolsulfonic acid) was observed to exert a specific inhibitory effect on U-87MG cells via various pathways. In addition, in cases of mixed compositions of pyrvinium/poly(cresolsulfonic acid) (200 nM/110 µg/mL and 250 nM/124 µg/mL) no significant difference in the cell viability was observed. The optimal concentration of the mixture was thus determined to be pyrvinium 200 nM and poly(cresolsulfonic acid) 110 µg/mL. Figure 3(c) shows a U-87MG cell cultured in a drug-free medium for 72 h; Figure 3(d) shows a photograph after incubation in a medium mixed with pyrvinium 200 nM and poly(cresolsulfonic acid) 110 µg/mL for 72 h. In the absence of drugs, it was confirmed that the bead-type cluster cell structure and the brain tumor cell-specific structure, which are distinct vine shaped structures, were observed. In the case of drugs, most of the bead-type and vine-shaped structures disappeared. Therefore, it is concluded that pyrvinium/poly(cresolsulfonic acid) mixture suppresses the function of cancer cells rather than causing cytotoxicity.
A Change in the State of Cells Upon Drug Removal and Re-drugging. Figure 4 shows the results of adding a mixture of pyrvinium 200 nM and poly(cresolsulfonic acid) 110 µg/mL, removing the drug from the U-87MG cells that were cultured for 3 days, incubating the cells for 3 days, and adding the drug again and incubating for 3 days. Figures 4(a) and 4(b) illustrate the findings stated in Section 3.4, and in case of cells that were cultured for 3 days after the drug was applied and the drug removed from the cells cultured for 3 days (Figure 4(c)), the appearance of bead-type clusters and a vine shape structure began to appear again. In that state, the drug was applied again and the cells were incubated for 3 days, and as a result of WST-8 assay, approximately 80% of the cells were killed (Figure 4(d)). The experiment attempted to determine the response to cells when the drug was removed and whether the drug was resistant to reapplication. It was a short experiment, but if the drug was removed, the cells tended to proliferate again, and if the drug was applied again, the degree of cell death increased, so it is highly likely that there was no resistance to the drug. Therefore, it will need to be applied to animal testing later.
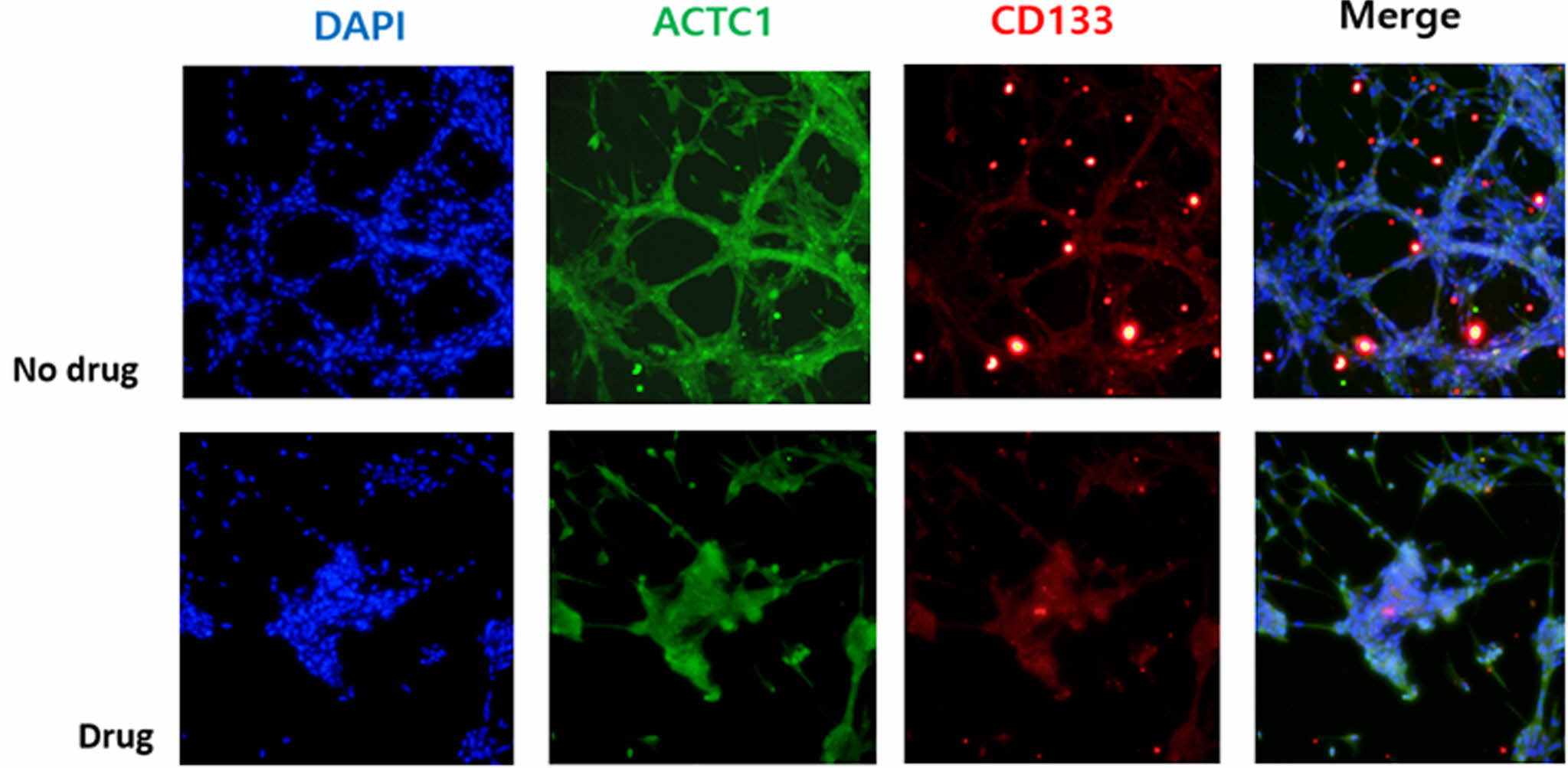
Analysis of ACTC-1 and CD133 Positive Cells. Previous studies have reported that the movement of GBM cells was inhibited when ACTC-1 (actin alpha cardiac muscle 1) was suppressed, and as CSCs, there have been reports of reduced drug resistance by the suppression of CD133 positive cells.15,16,32 Therefore, immunochemical staining was performed to confirm the hypothesis of cell proliferation and morphological change by synthesizing the previous results in this study. A mixture of pyrvinium 200 nM and poly(cresolsulfonic acid) 110 µg/mL was added and the cells were incubated for 3 days; additionally, immunochemical staining of ACTC-1 and CD133 was attempted for comparison with the medium without drug addition. As shown in Figure 5, the expression of ACTC-1 and the amount of CD133 positive cells were reduced, thereby inhibiting the formation of cell structures with aggressive vine-shaped infiltration characteristics.
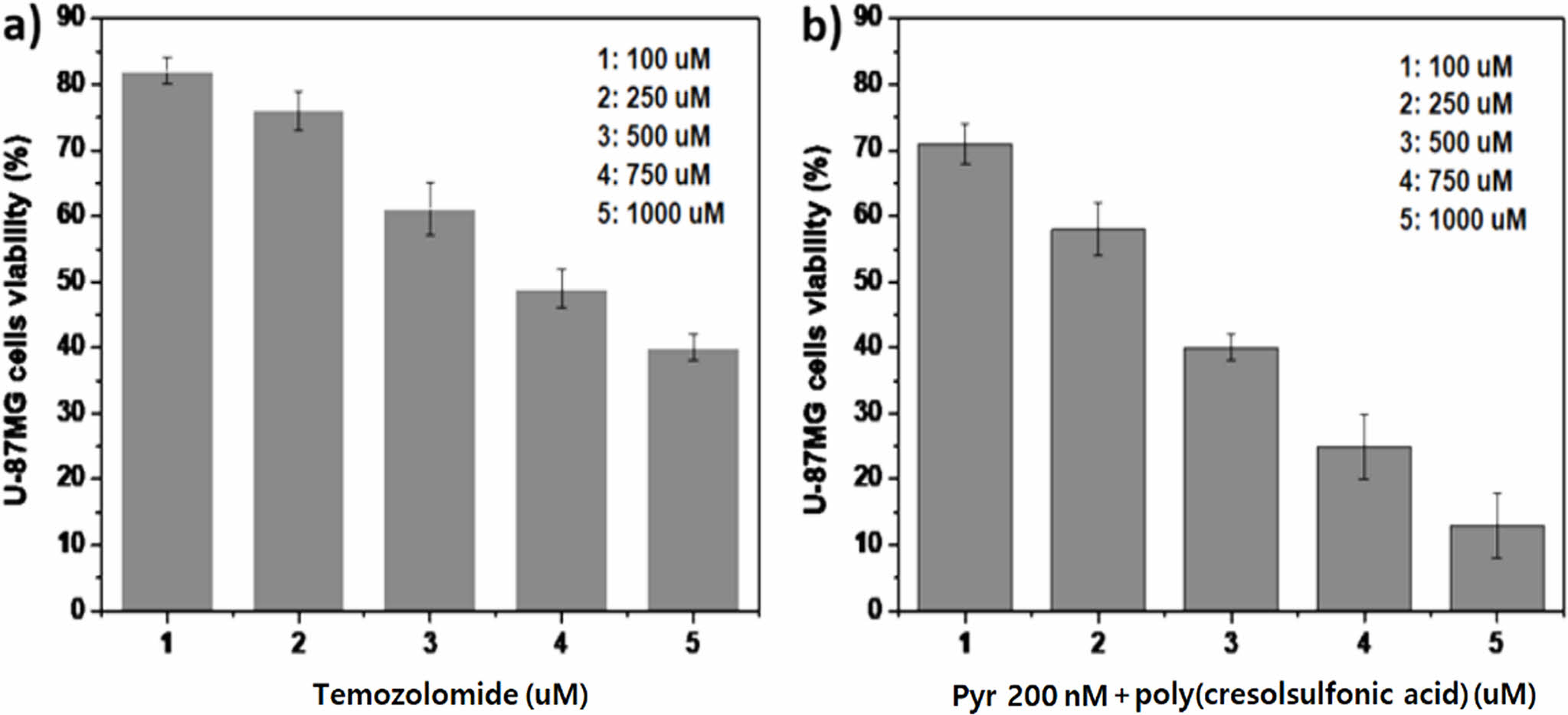
A Study on the Cell Responses in Temozolomide and Pyrvinium and Poly(Cresolsulfonic Acid) Mixture. Cell proliferation was observed by increasing the concentration of TZM in a mixture of pyrvinium 200 nM and poly(cresolsulfonic acid) 110 µg/mL. As shown in Figure 6(a), when only TMZ, a cytotoxic anticancer drug, was applied, the concentration inhibiting the proliferation of approximately 50% of cells was approximately 700-750 µM. Additionally, cytotoxicity was exhibited at a significantly high concentration of TMZ as a drug approved for GBM. In contrast, in the case of the mixture (Figure 6(b)), a cell inhibition of 50% showed a tendency to decrease significantly to approximately 300-350 µM. Poly(cresolsulfonic acid), pyrvinium, and temozolomide all play different roles. It is believed to be the result of the combination of inhibition of selective energy metabolism of CD-133 positive cells by firbinium, cytotoxic effect by guanine methylation reaction of temozolomide, and ECM inhibition effect of poly(cresolsulfonic acid). Considering that the cytotoxicity of the mixture is high, research on combination administration with TMZ is needed in the future.
A Study on the Response of Liver and Pancreatic Cancer Cells Using Pyrvinium/Poly(Cresolsulfonic Acid) Mixture. As previously described in Section 2.5, the cell responses to pancreatic (AsPC-1) and liver (HepG2) cancers were tested using a mixture of pyrvinium and poly(cresolsulfonic acid) of various concentrations. As shown in Table 3, cytotoxicity and similar morphological changes as in U-87 MG cells were observed in proportion to drug concentration in the liver cancer cell group, while no effect in terms of cell morphological changes and proliferation was observed in the pancreatic cancer cell group. Therefore, the results of U-87MG and HepG2 were completely different from AsPC-1 cells, possibly due to a difference in the mechanism by which the drug reacts within the cell. This particular aspect needs further research, and it has been confirmed that it responds specifically to U-87MG and HepG2 cells.
|
Figure 2 Results of culturing cells in a medium containing 0, 1, 5, and 10% FBS: (a) U-87MG; (b) NIH-3T3. |
|
Figure 3 Results of observing the response of cells while increasing the amount of pyrvinium and poly(cresolsulfonic acid): (a) U87MG; (b) NIH-3T3; (c) U-87MG image (×100) cultured without drugs; (d) U-87 MG image cultured with drugs (×100) and low magnification (×40) photograph on the upper right side of the photograph, (1: 50/28, 2: 75/41, 3: 100/55, 4: 125/69, 5: 150/83, 6: 175/ 97, 7: 200/110, and 8: 250 nM/124 µg/mL as poly(cresolsulfonic acid)/pyrvinium mixtures). |
|
Figure 4 Photographs of the cells after drugged, drug removal and re-drugging: (a) U-87MG image cultured without drugs; (b) U-87MG image cultured with mixture of pyrvinium 200 nM and poly(cresolsulfonic acid) 110 µg/mL; (c) cultured image for 3 days after removing the drug from the U-87MG cells; (d) cultured image for 3 days after adding the drug again from Figure 3(c), ×100. |
|
Figure 5 Results of immunostaining on ACTC-1 and CD133 positive cells. |
|
Figure 6 Results of cell viability in temozolomide and pyrvinium and poly(cresolsulfonic acid) mixture: (a) cell survival rate when using temozolomide alone; (b) cell survival rate when using mixtures (temozolomide and pyrvinium and poly(cresolsulfonic acid)). |
|
Table 1 Comparison of Cells Viability of Cells After 96 hours of Incubation Under Varying Concentrations of Pyvrinium |
|
Table 2 Comparison of Cells Viability of Cells After 96 Hours of Incubation Under Varying Concentrations of Poly(cresolsulfonic Acid) |
|
Table 3 Liver and Pancreatic Cancer Cells Viability (%) Using Pyrvinium/Poly(cresolsulfonic Acid) Mixture |
The effect of a mixture of pyrvinium and poly(cresolsulfonic acid) on GBM cells, which are malignant brain tumor cells, was evaluated. An analysis of the cell morphological changes and survival rates as well as the ACTC-1 and CD133 positive cells was conducted, and the formation of cancer-specific invasive and bead-type cell clusters were confirmed to be suppressed. Additionally, the cytotoxicity increased significantly when the mixture was used in combination with TMZ than when used alone. In future animal experiments, research on carriers for blood-brain barrier passage should be preceded.
- 1. <!--[if !supportLists]-->1. <!--[endif]-->Duwa, R.; Emami, F.; Lee, S.; Jeong, J. H.; Yook, S. Polymeric and Lipid-based Drug Delivery Systems for Treatment Ofglio- blastoma Multiforme. J. Ind. Eng. Chem. 2019, 79, 261-273.
-

- 2. <!--[if !supportLists]-->2. <!--[endif]-->Tran, K.; Brice, R.; Yao, L. Bioscaffold-based Study of Glioblastoma Cell Behavior and Drug Delivery for Tumor Therapy. Neurochem. Int. 2021, 147, 105049-105060.
-

- 3. <!--[if !supportLists]-->3. <!--[endif]-->Hill, L.; Bruns, J.; Zustiak, S. P. Hydrogel Matrix Presence and Composition Influence Drug Responsesof Encapsulated Glio- blastoma Spheroids. Acta Biomater. 2021, 132, 437-447.
-

- 4. <!--[if !supportLists]-->4. <!--[endif]-->Bastiancich, C.; Malfanti, A.; Préat, V.; Rahman, R. Rationally Designed Drug Delivery Systems for the Local Treatment of Resected Glioblastoma. Adv. Drug Deliv. Rev. 2021, 177, 113951-113977.
-

- 5. <!--[if !supportLists]-->5. <!--[endif]-->Lia, Y.; Marcu, L. G.; Hull, A.; Bezak, E. Radioimmunotherapy of Glioblastoma Multiforme - Current Status and Future Prospects. Crit. Rev. Oncol. Hematol. 2021, 163, 103395.
-

- 6. <!--[if !supportLists]-->6. <!--[endif]-->Guo, L.; Li, X.; Chen, Y.; Liu, R.; Ren, C.; Du, S. Radiotherapy (HFRT) with Concurrent and Adjuvant Temozolomide in Newly Diagnosed Glioblastoma: A Meta-analysis. Cancer Radiother. 2021, 25, 182-190.
-

- 7. <!--[if !supportLists]-->7. <!--[endif]-->Gramatzki, D.; Roth, P.; Rushing, E. J.; Weller, J.; Andratschke, N.; Hofer, S.; Korol, D.; Regli, L.; Pangalu, A.; Pless, M.; Oberle, J.; Bernays, R.; Moch, H.; Rohrmann, S.; Weller, M. Bevacizumab May Improve Quality of Life, But Not Overall Survival in Glioblastoma: An Epidemiological Study. Ann. Oncol. 2018, 29, 1431-1436.
-

- 8. <!--[if !supportLists]-->8. <!--[endif]-->Bregy, A.; Wong, T. M.; Shah, A. H.; Goldberg, J. M.; Komotar, R. J. Active Immunotherapy Using Dendritic Cells in the Treatment of Glioblastoma Multiforme. Cancer Treat. Rev. 2013, 39, 891-907.
-

- 9. <!--[if !supportLists]-->9. <!--[endif]-->Sivoria, S.; Parolini, S.; Marcenaro, E.; Castriconi, R.; Pende, D.; Millo, R.; Moretta, A. Involvement of Natural Cytotoxicity Receptors in Human Natural Killer Cell-mediated Lysis of Neuroblastoma and Glioblastoma Cell Lines. J. Neuroimmunol. 2000,107, 220-225.
-

- 10. <!--[if !supportLists]-->10. <!--[endif]-->Tunici, P.; Gianni, D.; Finocchiaro, G. Gene Therapy of Glio- blastomas: From Suicide to Homicide. Prog. Brain Res. 2001, 132, 711-719.
-

- 11. <!--[if !supportLists]-->11. <!--[endif]-->Ohnishi, K.; Tani, T.; Tojo, N.; Sagara, J. Glioblastoma Cell Line Shows Phenotypes of Cancer Stem Cells in Hypoxic Micro- environment of Spheroids. Biochem. Biophys. Res. Commun. 2012, 546, 150-154.
-

- 12. <!--[if !supportLists]-->12. <!--[endif]-->Berthier, S.; Arnaud, J.; Champelovier, P.; Col, E.; Garrel, C.; Cottet, C.; Boutonnat, J.; Laporte, F.; Faure, P.; Hazane-Puch, F. Anticancer Properties of Sodium Selenite in Human Glioblastoma Cell Cluster Spheroids. J. Trace Elem. Med. Biol. 2017, 44, 161-176.
-

- 13. <!--[if !supportLists]-->13. <!--[endif]-->Noh, K. H.; Lee, S. H.; Lee, H.; Jeong, A. J.; Kim, K. O.; Shin, H. M.; Kim, H. R.; Park, M. J.; Park, J. B.; Lee, J.; Ye, S. K. Novel Cancer Stem Cell Marker MVP Enhances Temozolomide-resistance in Glioblastoma. Transl. Oncol. 2021, 15, 101255.
-

- 14. <!--[if !supportLists]-->14. <!--[endif]-->Werner, M.; Lyu, C.; Stadlbauer, B.; Schrader, I.; Buchner, A.; Stepp, H.; Sroka, R.; Pohla, H. The Role of Shikonin in Improving 5-Aminolevulinic Acid-based Photodynamic Therapy and Chemotherapy on Glioblastoma Stem Cells. Photodiagnosis Photodyn. Ther. 2022, 26, 102987.
-

- 15. <!--[if !supportLists]-->15. <!--[endif]-->Kim, J. S.; Shin, D. H.; Kim, J. S. Dual-targeting Immunolipo- somes Using Angiopep-2 and CD133 Antibody for Glioblastoma Stem Cells. J. Control Release 2018, 269, 245-257.
-

- 16. <!--[if !supportLists]-->16. <!--[endif]-->Jamal, M.; Rath, B. H.; Tsang, P. S.; Camphausen, K.; Tofilon, P. J. The Brain Microenvironment Preferentially Enhances the Radioresistance of CD133+ Glioblastoma Stem-like Cells. Neoplasia 2012,14, 150-158.
-

- 17. <!--[if !supportLists]-->17. <!--[endif]-->Matias, D.; Predes, D.; Niemeyer Filho, P.; Lopes, M. C.; Abreu, J. G.; Lima, F. R. S.; Moura Neto, V. Microglia-glioblastoma interactions: New role for Wnt signaling. Biochim. Biophys. Acta - Rev. Cancer 2017, 1868, 333-340.
-

- 18. <!--[if !supportLists]-->18. <!--[endif]-->Khan, M.; Muzumdar, D.; Shiras, A. Attenuation of Tumor Suppressive Function of FBXO16 Ubiquitin Ligase Activates Wnt Signaling In Glioblastoma. Neoplasia 2019, 21, 106-116.
-

- 19. <!--[if !supportLists]-->19. <!--[endif]-->Tompa, M.; Kajtar, B.; Galik, B.; Gyenesei, A.; Kalman, B. DNA Methylation and Protein Expression of Wnt Pathway Markers in Progressive Glioblastoma. Pathol. Res. Pract. 2021, 222, 153429.
-

- 20. <!--[if !supportLists]-->20. <!--[endif]-->Lu, C.; Cui, C.; Liu, B.; Zou, S.; Song, H.; Tian, H.; Zhao, J.; Li, Y. FERMT3 Contributes to Glioblastoma Cell Proliferation and Chemoresistance to Temozolomide Through Integrin Mediated Wnt Signaling. Neurosci. Lett. 2017, 657, 77-83.
-

- 21. <!--[if !supportLists]-->21. <!--[endif]-->Bagca, B. G.; Ozates, N. P.; Asik, A.; Caglar, H. O.; Gunduz, C.; Avci, C. B. Temozolomide Treatment Combined with AZD3463 Shows Synergisticeffect in Glioblastoma Cells. Biochem. Biophys. Res. Commun. 2020, 533, 1497-1504.
-

- 22. <!--[if !supportLists]-->22. <!--[endif]-->Venugopal, C.; Hallett, R.; Vora, P.; Manoranjan, B.; Mahendram, S.; Qazi, M. A.; McFarlane, N.; Subapanditha, M.; Nolte, S. M.; Singh, M.; Bakhshinyan, D.; Garg, N.; Vijayakumar, T.; Lach, B.; Provias, J. P.; Reddy, K.; Murty, N. K.; Doble, B. W.; Bhatia, M.; Hassell, J. A.; Singh, S. K. Pyrvinium Targets CD133 in Human Glioblastoma Brain Tumor-Initiating Cells. Clin. Cancer Res. 2015, 21, 5324-5337.
-

- 23. <!--[if !supportLists]-->23. <!--[endif]-->Li, H.; Liu, S.; Jin, R.; Xu, H.; Li, Y.; Chen, Y.; Zhao, G. Pyrvinium Pamoate Regulates MGMT Expression Through Suppressing the Wnt/β-catenin Signaling Pathway to Enhance the Glioblastoma Sensitivity to Temozolomide. Cell Death Discov. 2021, 7, 288.
-

- 24. <!--[if !supportLists]-->24. <!--[endif]-->Biswas, A.; Yetirajam, R.; Das, S.; Parekh, A.; Mand, M. Targeting ECM via Stress Associated Regulation: A Potential Therapeutic Avenue for Overcoming Temozolamide Resistance in Glioblastoma. New Biotechnol. 2018, 44, S141-S142.
-

- 25. <!--[if !supportLists]-->25. <!--[endif]-->Zhong, J.; Shan, W.; Zuo, Z. Norepinephrine Inhibits Migration and Invasion of Human Glioblastoma Cell Cultures Possibly via MMP-11 Inhibition. Brain Res. 2021, 1756, 147280.
-

- 26. <!--[if !supportLists]-->26. <!--[endif]-->Pal, M.; Bhattacharya, S.; Kalyan, G.; Hazra, S. Cadherin Profiling for Therapeutic Interventions in Epithelial Mesenchymal Transition (EMT) and Tumorigen. Exp. Cell Res. 2018, 368, 137-146.
-

- 27. <!--[if !supportLists]-->27. <!--[endif]-->Lewis-Tuffin, L. J.; Rodriguez, F.; Giannini, C.; Scheithauer, B.; Necela, B. M.; Sarkaria, J. N.; Anastasiadis, P. Z. Misregulated E-Cadherin Expression Associated with an Aggressive Brain Tumor Phenotype. PLoS ONE 2010, 5, e13665-13676.
-

- 28. <!--[if !supportLists]-->28. <!--[endif]-->Noronha, C.; Ribeiro, A. S.; Taipa, R.; Castro, D. S.; Reis, J.; Faria, C.; Paredes, J. Cadherin Expression and EMT: A Focus on Gliomas. Biomedicines 2021, 9, 1328.
-

- 29. <!--[if !supportLists]-->29. <!--[endif]-->Espitia De La Hoz, F. Efficacy and Tolerance of Policresulen in the Treatment of the Genitourinary Syndrome of Menopause. Int. J. Family Community Med. 2019, 3, 132-136.
-

- 30. <!--[if !supportLists]-->30. <!--[endif]-->Zaher, A. R.; Helal, M. E.; Grawish, M. E.; Zedan, W. Immunohistochemical Expression of Epithelial Cadherin in the Buccal Mucosa of Guinea Pigs After Topical Application of Policresulen. Cairo Dental Journal 2014, 20, 17-21.
- 31. <!--[if !supportLists]-->31. <!--[endif]-->Junior, I. F.; Kotze, P. G.; Rocha, J. G.; Miranda, E. F.; Sartor, M. C.; Martins, J. F.; Rejaile, V. A.; Filho, A. S.; Correa, M. F. Postoperative Topical Analgesia of Hemorrhoidectomy with Policresulen and Cinchocaine: A Prospective and Controlled Study. Rev. Col. Bras. Cir. 2014, 41, 92-98.
-

- 32. 32. <!--[endif]-->Ohtaki, S.; Wanibuchi, M.; Kataoka-Sasaki, Y.; Sasaki, M.; Oka, S.; Noshiro, S.; Akiyama, Y.; Mikami, T.; Mikuni, N.; Kocsis, J. D.; Honmou, O. ACTC1 as An Invasion and Prognosis Marker in Glioma. J. Neurosurg. 2017, 126, 467-475.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2022 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2023; 47(3): 379-386
Published online May 25, 2023
- 10.7317/pk.2023.47.3.379
- Received on Feb 8, 2023
- Revised on Mar 12, 2023
- Accepted on Mar 13, 2023
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
- Conflict of Interest
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Jin Ik Lim
-
Department of Chemical Engineering, College of Engineering, Dankook University, Jukjeon-dong, Yongin-si, Gyeonggi-do 16890, Korea
- E-mail: limjinik@dankook.ac.kr
- ORCID:
0000-0003-4803-0455










 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.