- Synthesizing Heat-resistant Activated Carbon Fibers Using Cellulose Fiber Wastes
Department of Environmental Engineering, Anyang University, Anyang 14028, Korea
- 활성탄소섬유 제조를 위한 셀룰로오스계 폐섬유의 열안정성 연구
안양대학교 환경에너지공학과
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
Activated carbon fibers exhibit high kinetic and isothermal properties, but their high production cost poses a challenge for wider use. We envision that recycling the used cellulose-based waste fibers into flame-retardant activated carbon fibers can bridge this gap. In this work, both physical and chemical activation were performed after modifying the carbonaceous surface with H3PO4, K2HPO4, or K3PO4 + H2O. We find that using H3PO4 alone is more effective than K3HPO4 or H3PO4 + H2O, whereupon the former showed better physicochemical properties.
활성탄소섬유는 높은 동역학 및 등온적 특성을 보이지만, 높은 생산 비용으로 인해 광범위한 사용에 어려움을 겪고 있다. 본 연구에서는 셀룰로오스 기반 폐섬유를 난연성 활성탄소섬유로 재활용하여 상기 기술과 경제성 간 발생하는 격차를 해소하는 것에 중점을 두었다. 폐섬유를 활성탄소섬유로 재활용함에 있어, 난연제로 잘 알려진 H3PO4, K2HPO4 및 K3PO4 + H2O를 통해 탄소질 표면을 개질하였다. 이후 물리적 및 화학적 활성화 공정을 실시하였다. 특성평가를 통해 난연제 활용이 활성탄소섬유 공정의 열안정성, 최종수율, 난연성, 및 미세기공율에 긍정적인 영향을 미치는 것을 확인하였다. 다양한 조성의 샘플군 중 H3PO4를 단독으로 사용하는 것이 K3HPO4나 H3PO4 + H2O를 적용하는 것 대비 상기 언급된 부분에서 더 나은 물리화학적 특성을 나타냈다.
Cellulose fiber wastes were recycled into activated carbon fibers and they were further treated with phosphoric acids (K2HPO4 or K3PO4 + H2O). H3PO4 was also treated onto activated carbon fibers as a control sample. We found that treating activated carbon fibers with H3PO4 is more effective than K3HPO4 or H3PO4 + H2O, whereupon the former revealed better physicochemical characteristics.
Keywords: activated carbon fiber, heat resistance, recycling, cellulose fiber wastes, surface modification.
We thank Korea Environmental Industry & Technology Institute (KEITI) for supporting our research (2022003160015).
The authors declare that there is no conflict of interest.
Information is available regarding the sample characteristics using Brunauer-Emmett-Teller and Brunauer-Joyner-Halenda methods. The materials are available via the internet at http://journal.polymer-korea.or.kr.
PK_2023_047_04_399_Supporting_Information_template.pdf (731 kb) Supplementary Information
Carbonaceous fibers have garnered significant attention in numerous research domains, including adsorption, high-strength materials, and as structural frameworks for reinforced plastics.1-5 The conventional production process for carbonaceous fibers entails spinning, dyeing, and processing, which necessitates substantial utilization of fossil fuels and chemicals. Consequently, these processes yield by-products such as microplastics, wastewater, and greenhouse gases, exacerbating environmental concerns. As a result, there has been a surge of interest in recycling carbonaceous fibers, giving rise to a burgeoning body of research.6
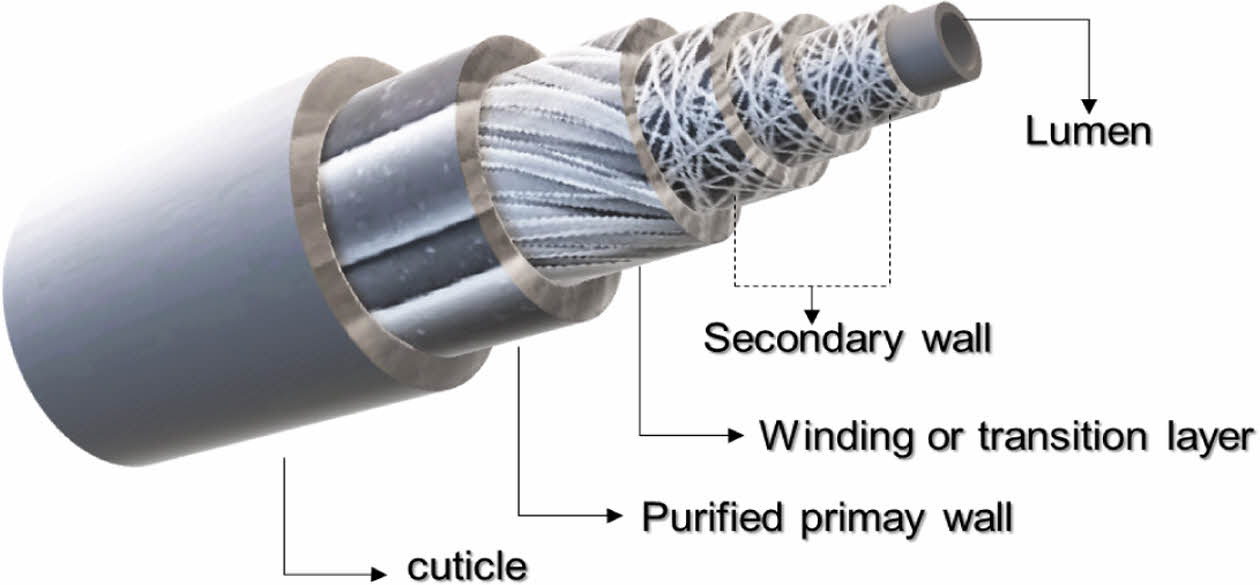
Of particular note are cellulose-based activated carbon fibers, which are fabricated from eco-friendly materials through an activation process. These fibers possess valuable properties, such as odor, heavy metal, and drug adsorption.7-10 The cellulose-based activated carbon fiber structure, delineated in Figure 1, comprises 90% cellulose, 10% moisture, and 0.5% protein. Crucial parameters for recycling cellulose-based activated carbon fibers include yield, thermal properties, and surface characteristics. Yield, defined as the difference in weight before and after manufacturing the activated carbon fiber, has a direct impact on overall production costs. The thermal properties of the fibers determine the range of potential applications, while surface characteristics relate to surface areas based on pore sizes, including micropores, mesopores, and macropores. A higher surface area for activated carbon fiber may result in enhanced kinetic parameters concerning adsorption.
In this study, waste cellulose fibers were repurposed into activated carbon fibers, which were subsequently treated with flame retardant to broaden their potential applications. The production of activated carbon fibers can be categorized into two distinct synthesis methods: physical and chemical. Physical synthesis involves varying carbonization and activation conditions, while chemical synthesis entails surface modification via catalysts or chemical crosslinks. This work adopted a combination of both physical and chemical synthesis to produce activated carbon fibers.
To determine the optimal conditions for flame retardant impregnation, samples were produced under various conditions, and the surface characteristics were monitored based on the concentration and attachment time of the flame retardant. Subsequently, the yield and thermal properties of the prepared sample were analyzed according to the identified optimal conditions. This study aims to contribute an eco-friendly alternative to the global activated carbon fiber market, where usage has exhibited a consistent upward trend on an annual basis.
|
Figure 1 Layout of typical cellulose fibers |
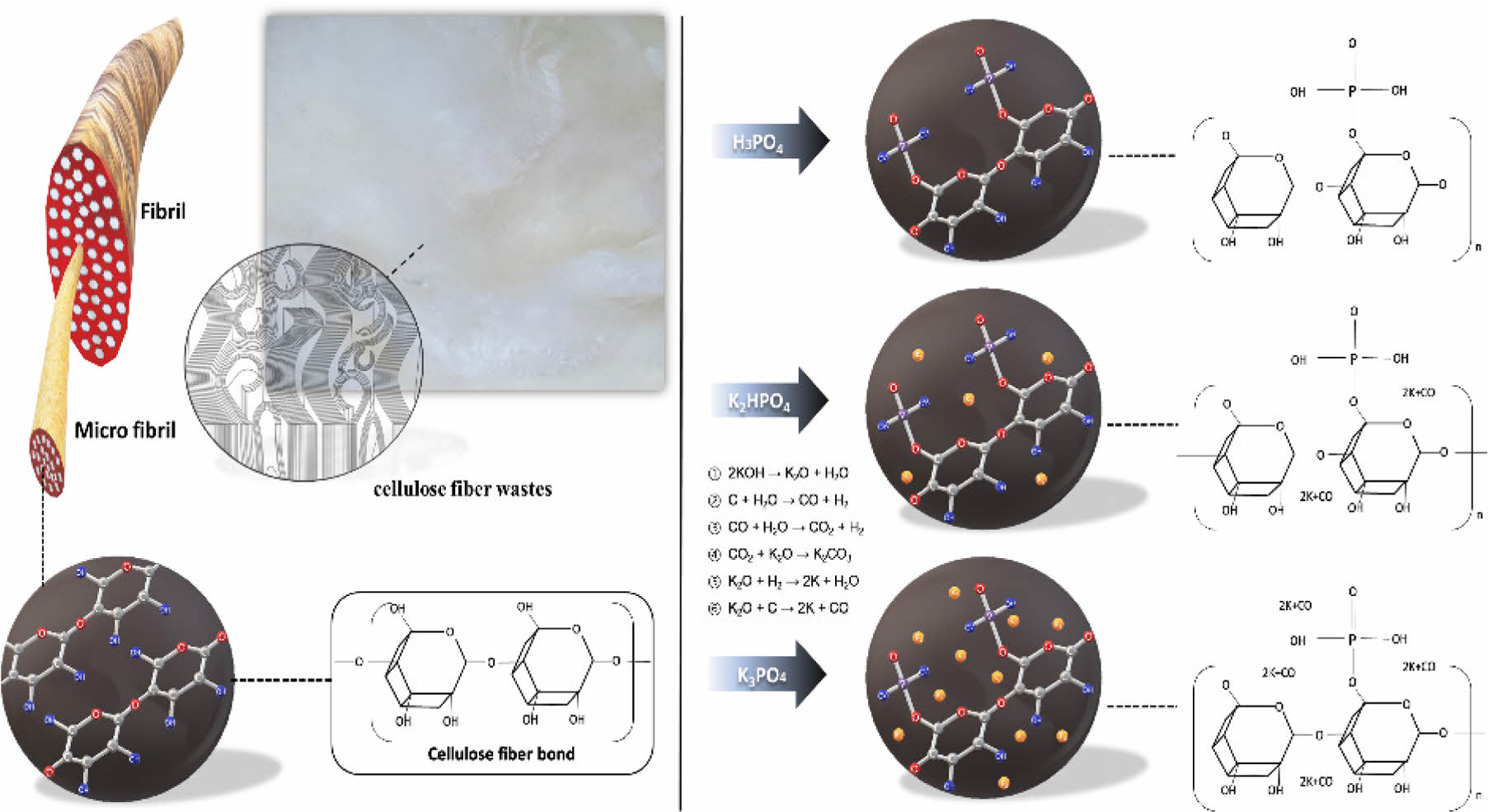
All chemical reagents utilized in this study were of analytical grade, procured from commercial suppliers (SK Chemical Daejung), and employed without further purification. The initial step to eliminate residual contaminants physically deposited on the waste fiber surface involved subjecting the samples to an extensive washing process, where pressure was applied to flowing water for a predetermined duration. Subsequently, K2HPO4 and K3PO4 in conjunction with water were employed as additives to confer heat resistant properties to the sample, while H3PO4 was utilized as a control group.
During the synthesis process of K2HPO4 and K3PO4 + H2O, KOH formation and deliquescence processes were initiated throughout the deposition and washing stages, which contributed to the enhancement of the micropore structure. In contrast, the application of H3PO4 induced char formation, consequently augmenting the thermal stability and flame retardancy of both the manufacturing process and the end products.11 To monitor the fiber yield concerning the concentration of the phosphoric acid-treated samples, the concentration was varied between 0.1 and 0.5 M. For H3PO4, concentrations ranging from 0.15 to 0.75 M were employed. The samples were immersed in three types of chemical solutions for 24 hr before being transferred to a desiccator for an additional 24 hr at 70 ℃.
The stabilization (oxidation) process was conducted at 260 ℃, employing a heating rate of 10 ℃ min-1 for a duration of 1 hr, to facilitate the elimination of impurities. During this stage, a dehydration reaction was triggered by stabilizing the sample at 260 ℃, leading to the chemical separation of water from hydrogen and hydroxy groups present within the sample. The preferential dissociation of C=O and C=C bonds occurred, followed by the decomposition of C-O and C-C bonds during the thermal decomposition process. The stabilized samples were subsequently placed in a desiccator for 24 hr at 70 ℃. The weight change was quantified, and the highest-yielding samples were selected from the three types of impregnation.
Carbonization was executed at a heating rate of 10 ℃ min-1 up to 700 ℃ in a nitrogen atmosphere for a period of 2 hr. The activation process followed, ranging from 0.5 to 3 hr, with the addition of water in a nitrogen gas atmosphere. To remove impurities from the finalized samples, they were washed with distilled water until a neutral pH of 7 was achieved. The samples were then placed in a desiccator for 24 hr at 70 ℃.
The surface characteristics of the dried samples were examined using the Brunauer-Emmett-Teller (BET) and Brunauer-Joyner-Halenda (BJH) methodologies, employing an ASAP2460 Micromeritics instrument. A scanning electron microscope (SEM) (S-3000H, HITACHI) was utilized to visually assess the impact of impurities on the samples. Lastly, the thermal attributes of the samples were analyzed through thermogravimetric analysis (TGA) using a TA Instruments Q500 device.
The oxygen index module equipment manufactured by YEONJIN S-Tech Corporation was used for the analysis of limited oxygen index (LOI).
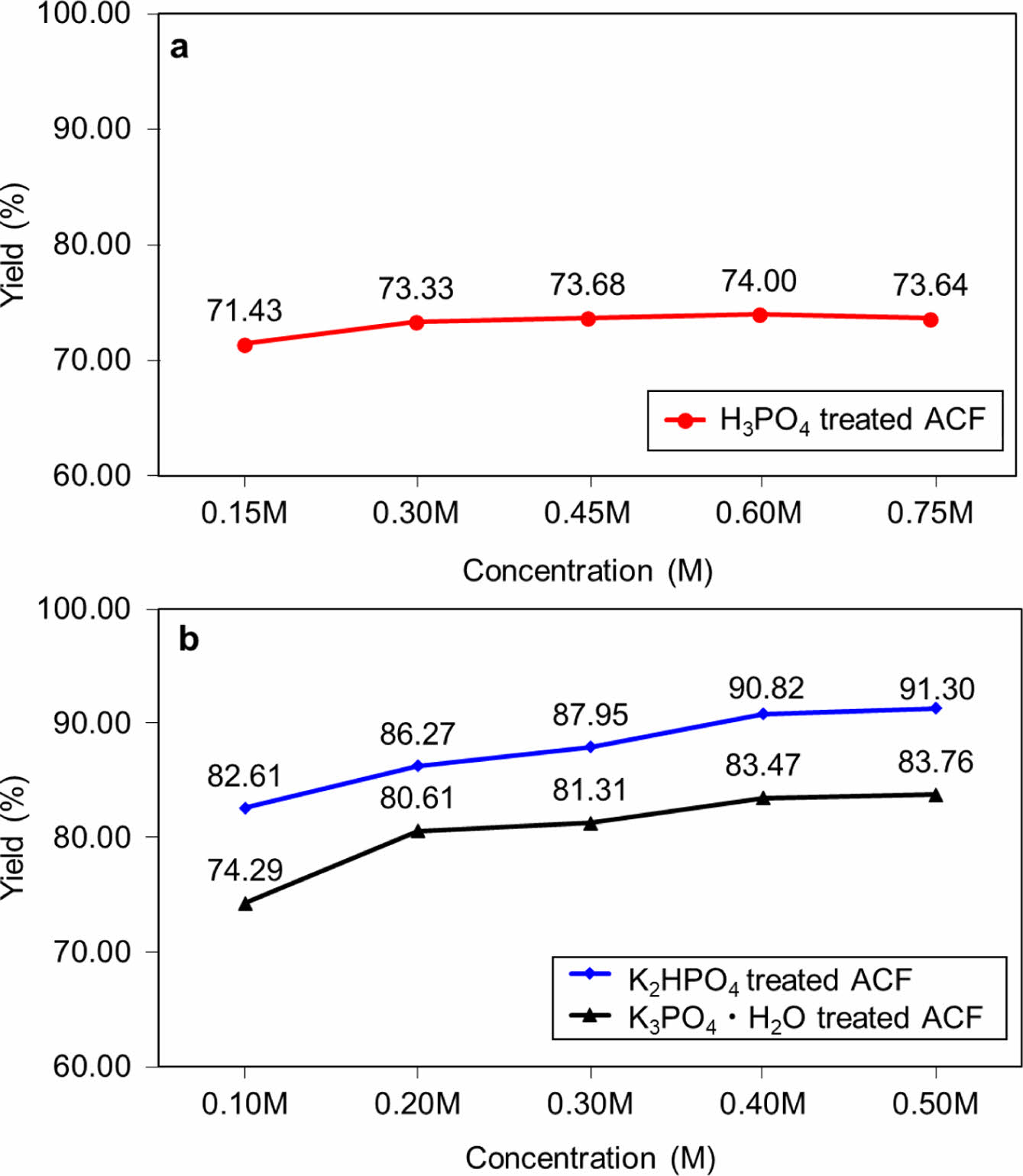
The yield of samples synthesized using three types of additives at different concentrations is shown in Figures 2. The yield of the samples was shown in the order of K2HPO4 > K3PO4 + H2O > H3PO4. In all cases, a relatively low yield was shown at a low concentration, but the yield tended to stabilize as the concentration increased. As the concentration of additives increases, the polyphosphate acid generated during the combustion of the phosphorus compound forms a protective layer on the sample surface (Figure S1(d)). In addition, dehydration is applied more quickly on the sample surface to block surrounding oxygen. In the impregnation process, the expected reaction rate according to the concentration was calculated as (Figures S1(a)-(c)) by substituting the initial concentration [A]0 for each sample (eq. (1)).

The expected reaction rate according to the concentration of each sample was in the order of H3PO4 > K2HPO4 = K3PO4 + H2O. In the case of H3PO4, it is believed that the competition between the PO4 and the sample surface was less than that of other samples, due to the absence of K content.
Carbonization and activation were proceeded using the highest yield of each sample; H3PO4 (0.6 M), K2HPO4 (0.5 M), and K3PO4 + H2O (0.5 M). The specific surface area and pore distribution were analyzed according to the BET method for activated samples (Figure S2). Here, an additional study was conducted before/after washing the sample to find out the physicochemical properties according to the influence of the remaining K content. In all cases, the 1 - 2 h interval was found to be the optimal impregnation time. In the case of samples excluding H3PO4, the surface variations before and after washing was significant. Particularly, in the case of a sample treated with K3PO4 + H2O, the BET surface area was found to be near 1m3g-1 before washing. This indicates that washing should be carried out essentially. The main reason is due to the dissociation of K content during the impregnation and reaction with H2O to form KOH. In addition, K content may be combined with OH- of cellulose to additionally form KOH. The KOH introduced through the two paths may block pores and eventually decrease BET surface areas.12-14 Such decrease in the BET surface area indicates a proportional decrease in micropores, mesopores, and macropores. This can also be seen from BJH data derived from the results of nitrogen gas adsorption isotherm analysis according to p·po-1 (relative pressure) at -196 ℃ using liquified nitrogen (Figure S3).
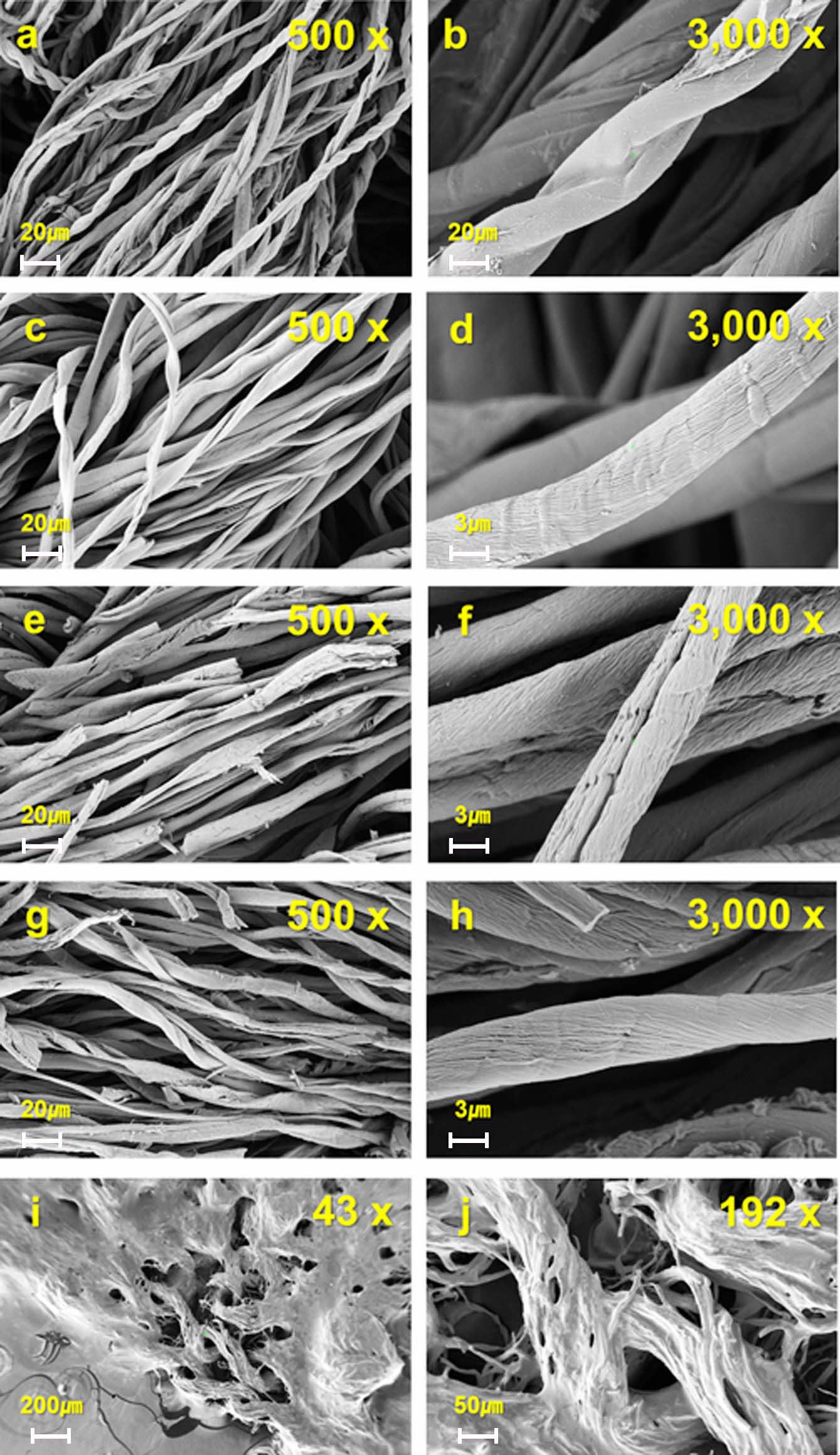
To support the numerical values of surface characteristics, samples were visually confirmed using SEM (Figure 3). Here, blank samples refer to cellulose fiber wastes. In all cases, observable damage was found on the surface of the chemically modified sample compared to the blank sample. Damages from the samples containing K content are mainly due to the newly introduced KOH. Non-washed samples seemed very limited for commercial use because they showed a loose shape beyond the fiber shape (Figures 3(i)-(j)). It is confirmed that designing flame-retardant activated carbon fibers by applying H3PO4 alone seems to be an effective method showing better physicochemical characteristics compared to other samples.
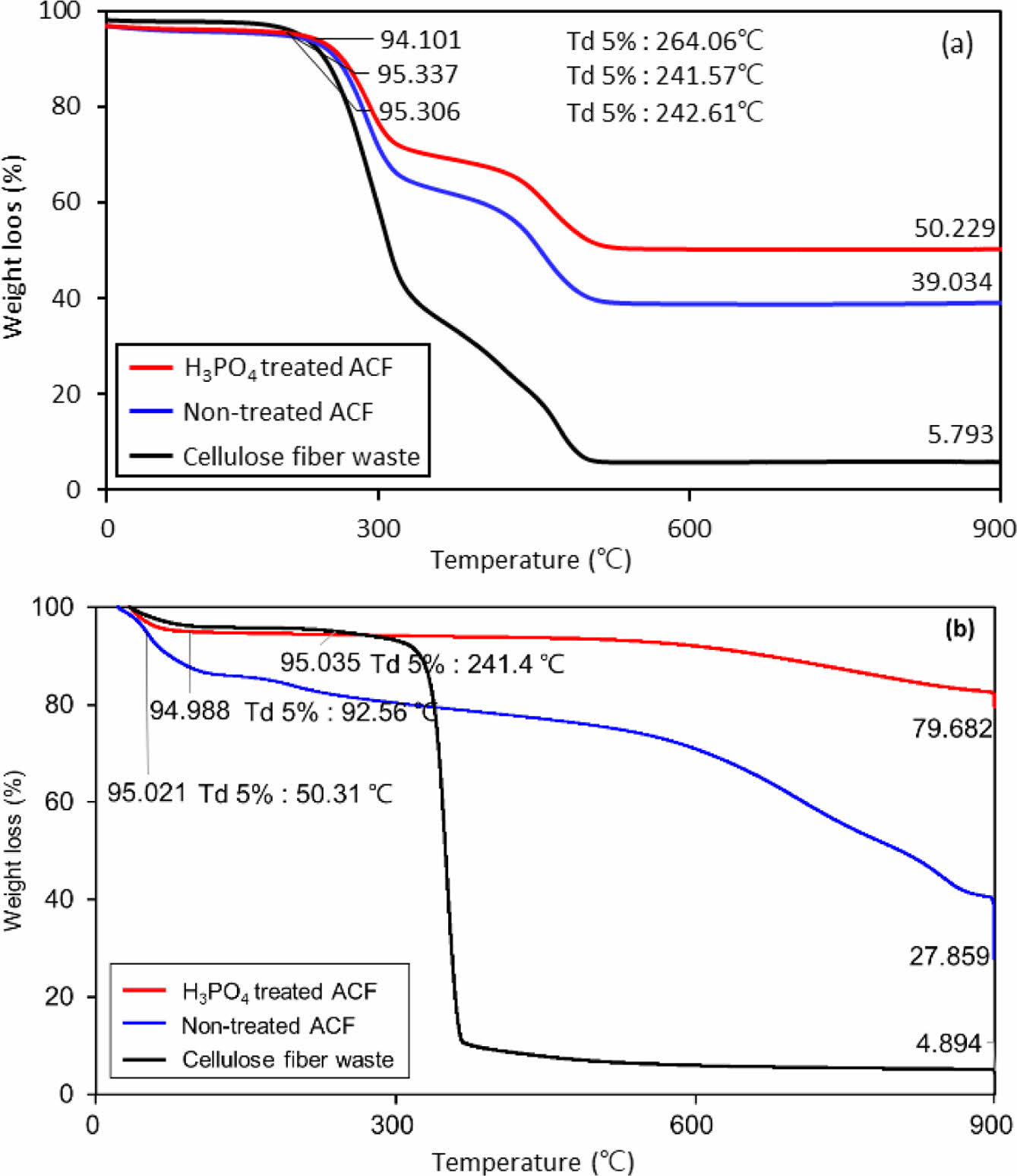
The flame retardancy of H3PO4 treated sample was studied via TGA in air and nitrogen atmosphere (Figure 4). The temperature increase rate was conducted at 10 ℃/min and hold for 5 hr at 900 ℃. The flame retardancy of activated carbon fiber and cellulose fiber waste was chosen as a control group. In case of nitrogen atmosphere, it was confirmed that the weight loss of the cellulose fiber waste decreased rapidly at 336.63 ℃, and decreased to less than 10% before 400 ℃. The residual weight after 5 hr at 900 ℃ was only 1.756 %. In the case of activated carbon fiber derived from the cellulose fiber waste, the residual weight after 5 hr at 900 ℃ was 17.786%. Lastly, a H3PO4 treated sample has secured more than 75% after 5 hr at 900 ℃. The results obtained from TGA showed that the flame retardancy was enhanced with the introduction of H3PO4.
In the case of air atmosphere, the weight loss of cellulosic fiber waste decreased sharply around 231.24 ℃, and it was found that it decreased by more than 10% before 300 ℃. After that, the residual weight was only 4.894%. On the other hand, in the case of activated carbon fiber using waste fiber, the weight after 900 ℃ residual is 39.034%, which is about 10% more than that of nitrogen atmosphere. However, the final residual weight of H3PO4-treated ACF was 50.229%, which is about 20% less than that of nitrogen atmosphere. Therefore, it was concluded that the carbonization activation process performed in nitrogen atmosphere has a higher final yield of fiber, and the thermal stability of H3PO4-treated ACF was confirmed.
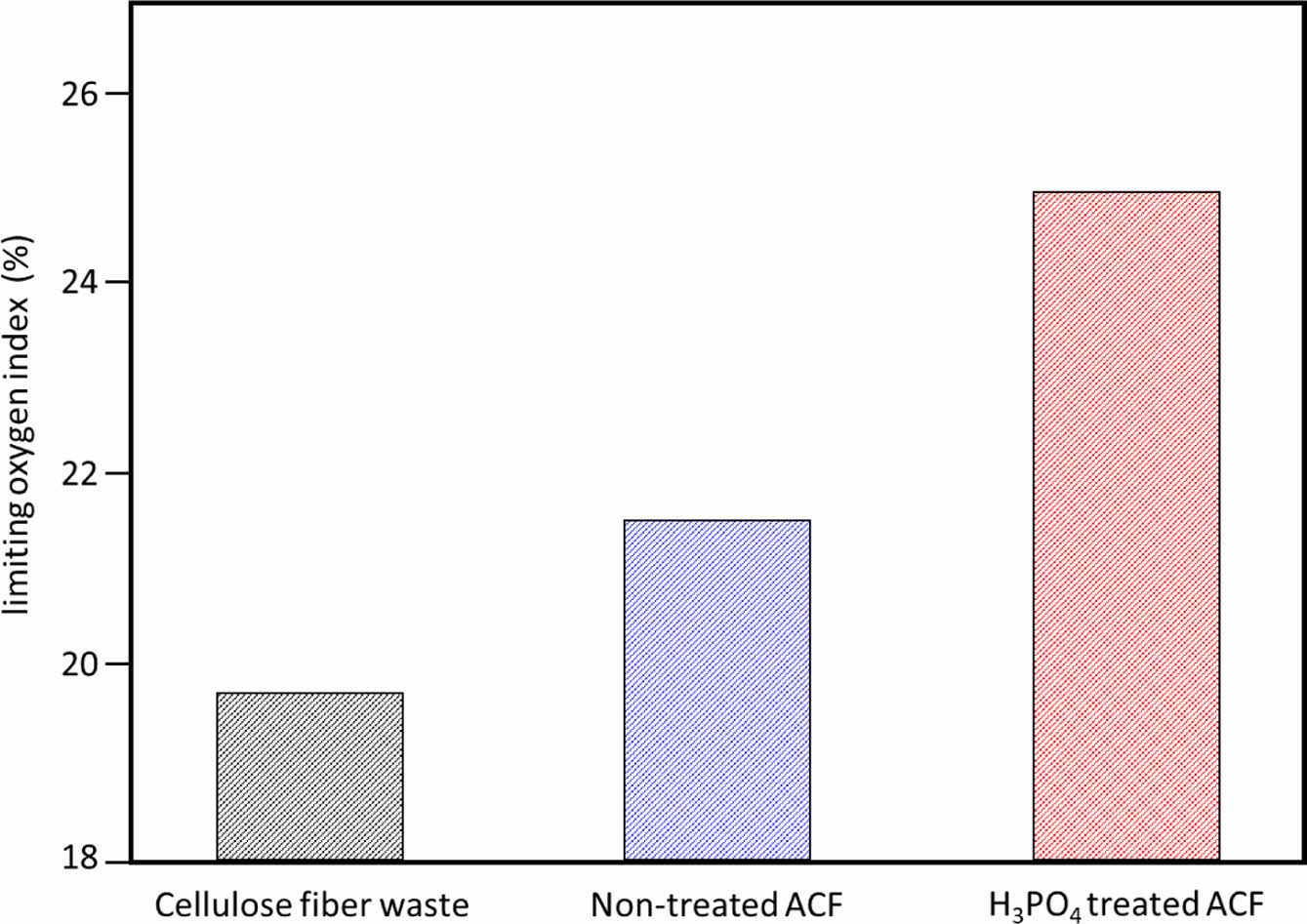
The limiting oxygen index (LOI) analysis was performed to determine the flame retardancy of each sample. The LOI, which represents the oxidation resistance of each sample, is calculated using eq. (2) and the corresponding results are shown in Figure 5.

In general, a LOI value below 17 is considered flammable and above 17 is considered flame retardant. All samples were in the flame retardant range, in the order of H3PO4 treated ACF > non-treated ACF > cellulose fiber waste. The oxygen index was found to be improved, and the LOI value of H3PO4 treated ACF increased by about 20% compared to cellulose fiber waste. It can be attributed that the addition of H3PO4 promotes the formation of char in the fiber and delays or inhibits combustion in the protective layer.
|
Figure 2 The yield of samples by various types of additives are shown in (a) and (b), respectively. |
|
Figure 3 Pictures of (a)-(b) blank, (c)-(d) H3PO4, (e)-(f) K2HPO4, (g)-(h) K3PO4 + H2O (i) non-washed K2HPO4, and (j) non-washed K3PO4 + H2O samples obtained via a scanning electron microscope. Here, the magnification of each picture is as follows: (a) × 500; (b) × 3000; (c) × 500; (d) × 3000; (e) × 500; (f) × 3000; (g) × 500; (i) × 43; (j) × 192. |
|
Figure 4 Thermogravimetric analyses of samples via (a) air; (b) nitrogen atmosphere. |
|
Figure 5 Limiting oxygen index (%) of cellulose fiber waste, Nontreated ACF, and H3PO4 treated ACF. |
H3PO4, K2HPO4, or K3PO4 + H2O were treated onto cellulose fiber wastes to design a heat resistant activated carbon fibers. On the one hand, the introduced K content resulted in a decrease in surface characteristics due to the pore-blocking of KOH. K2HPO4 or K3PO4 + H2O treated samples revealed lower BET surface areas and micropores. On the other hand, H3PO4 acted as a chemical shield onto the co-continuous structure of activated carbon fibers. H3PO4 treated sample was found to be suitable for applications such as high-heat resistant polymer at 900 ℃ for 5 hr of operating time. These results provide substantial motivation to continue the development of activated carbon fibers based on widely abundant wastes.
- 1. López-Salas, N.; Antonietti M. Carbonaceous Materials: the Beauty of Simplicity. Bull Chem. Soc. Jpn. 2021, 94, 2822-2828.
-

- 2. Lee, J. H.; Lee, S. H.; Suh, D. H. CO2 Treatment of Carbon Fibers Improves Adsorption of Fuel Cell Platinum. Environ. Chem. Lett. 2021, 19, 1809-1814.
-

- 3. Gong, Y.; Liao, M. D.; Liu, P.; Jin, Y. Z.; Chen, J.; Tang, Y. J. Large-scale Synthesis of Carbon Fiber Sponges by Chemical Vapor Deposition. Chem. Lett. 2020, 49, 542-545.
-

- 4. Lee, J. H.; Sim, S. J.; Kang, J. H.; Choi, S. S. Isotherm and Thermodynamic Modelling of Malachite Green on CO2-activated Carbon Fibers. Chem. Phys. Lett. 2021, 780, 138962.
-

- 5. Singh, G.; Lee, J. M.; Kothandam, G.; Palanisami, T.; Al-Muhtaseb, A. A. H.; Karakoti, A.; Yi, J.; Bolan, N.; Vinu, A. A Review on the Synthesis and Applications of Nanoporous Carbons for the Removal of Complex Chemical Contaminants. Bull. Chem. Soc. Jpn. 2021, 94, 1232-1257.
-

- 6. Rani, M.; Choudhary, P.; Krishnan, V.; Zafar, S. A Review on Recycling and Reuse Methods for Carbon Fiber/glass Fiber Composites Waste from Wind Turbine Blades. Compos. B. Eng. 2021, 215, 108768.
-

- 7. Bengtsson, A.; Bengtsson, J.; Sedin, M.; Sjöholm, E. Carbon Fibers from Lignin-cellulose Precursors: Effect of Stabilization Conditions. ACS Sustain. Chem. Eng. 2019, 7, 8440-8448.
-

- 8. Hassan, M. F.; Sabri, M. A.; Fazal, H.; Hafeez, A.; Shezad, N.; Hussain, M. Recent Trends in Activated Carbon Fibers Production from Various Precursors and Applications—A Comparative Review. J. Anal. Appl. Pyrolysis. 2020, 145, 104715.
-

- 9. Awad, R.; Mamaghani, A. H.; Boluk, Y.; Hashisho, Z. Synthesis and Characterization of Electrospun PAN-based Activated Carbon Nanofibers Reinforced with Cellulose Nanocrystals for Adsorption of VOCs. Chem. Eng. J. 2021, 410, 128412.
-

- 10. Dong, Y. D.; Zhang, H.; Zhong, G. J.; Yao, G.; Lai, B. Cellulose/carbon Composites and Their Applications in Water Treatment–a Review. Chem. Eng. J. 2021, 405, 126980.
-

- 11. Choi, S. S.; Lee, J. H.; Lee, S. H. Thermal Properties of Lyocell Fibers by Activation Energy and Pretreatment During Oxidation. Polym. Korea 2019, 43, 872-878.
-

- 12. Liu, L.; Li, Y.; Fan, S. Preparation of KOH and H3PO4 Modified Biochar and Its Application in Methylene Blue Removal from Aqueous Solution. Processes. 2019, 7, 891.
-

- 13. Jeguirim, M.; Belhachemi, M.; Limousy, L.; Bennici, S. Adsorption/reduction of Nitrogen Dioxide on Activated Carbons: Textural Properties Versus Surface Chemistry–a Review. Chem. Eng. J. 2018, 347, 493-504.
-

- 14. Sher, F.; Iqbal, S. Z.; Albazzaz, S.; Ali, U.; Mortari, D. A.; Rashid, T. Development of Biomass Derived Highly Porous Fast Adsorbents for Post-combustion CO2 Capture. Fuel. 2020, 282, 118506.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2022 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2023; 47(4): 399-403
Published online Jul 25, 2023
- 10.7317/pk.2023.47.4.399
- Received on Nov 29, 2022
- Revised on Apr 18, 2023
- Accepted on Apr 24, 2023
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
- Conflict of Interest
- Supporting Information
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Sang Sun Choi
-
Department of Environmental Engineering, Anyang University, Anyang 14028, Korea
- E-mail: choiss@anyang.ac.kr
- ORCID:
0000-0002-3133-7808










 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.