- Effects of Maleic Anhydride Content on Thermomechanical and Mechanical Properties of Maleated Natural Rubber
Nabil Hayeemasae*, **
 , Mohamad Irfan Fathurrohman***
, Mohamad Irfan Fathurrohman***  , and Abdulhakim Masa**, ****,†
, and Abdulhakim Masa**, ****,† 
*Department of Rubber Technology and Polymer Science, Faculty of Science and Technology, Prince of Songkla University, Pattani Campus, Pattani, 94000, Thailand
**Research Unit of Advanced Elastomeric Materials and Innovations for BCG Economy (AEMI), Faculty of Science and Technology, Prince of Songkla University, Pattani Campus, Pattani, 94000, Thailand
***Indonesian Rubber Research Institute, Salak Street No. 1 Bogor, 16128, Indonesia
****Rubber Engineering & Technology Program, International College, Prince of Songkla University, Hat Yai, Songkhla, 90110, Thailand- 무수 말레이산 함량에 따른 말레인산 천연고무의 열적 기계적 물성 연구
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
Influences of maleic anhydride (MA) contents (1-10 wt%) on the properties of natural rubber (NR) grafted with MA – or maleated natural rubber (MNR) – were investigated. According to rheometric tests, the scorch and cure times of MNRs were longer than that of NR, because the acidity of MA slowed down crosslinking reactions and decreased grafting efficiency of MA onto the NR. The maximum torque and torque difference of MNRs were improved over NR due to the maleated linkages formation. These maleated linkages induced greater crosslink density and shifted thermo-stability of NR toward higher temperature. A dramatic increase in crosslink density was attained in the MNR containing 1% MA, having also the highest thermomechanical properties and tensile strength. The thermostability and tensile strength of the MNR containing 1% MA were improved by about 10 ℃ and 53% over those of the NR.
Influences of maleic anhydride (MA) content on the properties of maleated natural rubber (MNR) were investigated. The maleated linkages forming in the MNRs during vulcanization induced greater crosslink density and tensile properties, and shifted thermo-stability of NR toward higher temperature. A dramatic improvement was attained in the MNR containing 1% MA, having also the highest thermomechanical properties and tensile strength.
Keywords: natural rubber, maleated natural rubber, grafting reaction, thermomechanical properties.
This research was supported by National Science, Research and Innovation Fund (NSRF) and Prince of Songkla University (Grant No. UIC6505044S).
The authors declare that there is no conflict of interest.
Maleated natural rubber (MNR) is a type of chemically modified natural rubber (NR) prepared by grafting maleic anhydride (MA) onto the NR. The chemical structure of MNR has MA attached as a pendant group to the NR polymer chains.1 The MNR, or NR that was grafted with MA (NR-g-MA), has been shown to have improved polarity over NR.2 This improved polarity allows MNR to be used as a blend compatibilizer in a variety of blend systems, for instance on blending NR with polypropylene,3 poly(methyl methacrylate),4 cassava starch,5 or paper sludge.6 Besides, the increased hydrophilicity from modifying NR also enhanced resistance to solvents, flex cracking, and ageing over typical NR vulcanizates.7
Two conventional techniques are used for grafting MA onto NR, namely either in the molten state with mixing conducted in an internal mixer at an elevated temperature above 135 ℃,8,9 or in a solution state with the rubber dissolved in a suitable solvent, such as toluene.10 However, the main drawbacks of melt-mixing approach include competitive reactions that result in a low level of grafting the MA onto the NR; whereas the need for a large amount of solvent, appropriate solvent disposal, and its impacts on environment are the main shortcomings of the solution technique. Owing to the fact that the latex form of NR is natural during its collection, chemical modification under ecologically friendly conditions, especially as a water suspension, is particularly intriguing. The grafting of MA onto NR was recently demonstrated for the latex form.2 This technique was found to be effective in synthesizing the MNR graft copolymers. Wongthong et al.,2 have modified NR via grafting with MA in latex state. The efficiencies of grafting MA onto neat NR or onto deproteinized NR (DPNR) latexes were compared. A greater grafting efficiency was achieved with DPNR, indicating that the proteins in NR latex were suppressing the grafting reactions. The maximum grafted content of MA was about 29%, which is much higher than what can be achieved with the other techniques, as the maximum contents from melt-mixing and solution techniques are about 3.5%10 and 7.5%, respectively.4
Although several studies have been conducted on the MNR, the majority of these were focused on the use of this modified rubber as a compatibilizer while less attention has been paid to the variation of MNR properties. The adhesion between two phases in a blend is generally improved by incorporating MNR as a compatibilizer, enhancing the mechanical properties of the final product.9,10 Since little attention has been paid to the characteristics of MNR itself, particularly the thermomechanical properties of MNR, a study on the influences of MA content on thermo-mechanical and mechanical properties of this rubber will address a knowledge gap. In this study, the grafting reaction was performed in the pure form of NR latex, even though the grafting efficacy was probably lower than that with DPNR.2 However, no additional chemical reagents and processing were required, as the main advantage of using pure NR latex over DPNR. The properties of the MNR with different MA contents were assessed with Fourier transform infrared spectrometry, as well as for curing, thermomechanical, and tensile properties.
Materials. Concentrated NR latex (HA) with 60% dry rubber content was purchased from Yala Latex Co., Ltd., Yala, Thailand, and was used to prepare maleated NR (MNR). Potassium hydroxide (85%), and isopropanol (99.5%) were purchased from KemAusTM (Australia). Sodium dodecyl sulfate (99%), maleic anhydride (99%), and benzoyl peroxide (75%) were purchased from Sigma-Aldrich (USA).
The rubber compounding ingredients stearic acid (Imperial Industrial Chemicals Co., Ltd., Thailand), zinc oxide (Global Chemical Co., Ltd., Thailand), wingstay L (Kij Paiboon Chemical Ltd., Thailand), N,N-dicyclohexyl-2-benzothiazole sulfonamide or DCBS (Flexsys America L.P., USA) and sulfur (Siam Chemical Industry Co., Ltd., Thailand) were used as received.
Preparation of MNR and MNR Compound Preparation. MNR was prepared using the latex state by adding various amounts of maleic anhydride (MA), i.e., 1, 3, 5, or 10 wt% (NR-MA 1, 3, 5, and 10) into the NR latex, according to the procedure described by Wongthong et al.2 However, the type of NR latex was not the same, e.g., pure form of the NR latex was used in this study. The mixture was then coagulated by using methanol, washed with deionized water and dried until constant weight in an oven at 70 ℃. Free NR was Soxhlet extracted with light petroleum ether for 24 h at 60 ℃, and ungrafted MA was removed by using acetone only as preparation for Fourier transform infrared spectrometry. The remaining MNRs were used as neat for compound preparation. The dried NR having various MA contents were sequentially compounded with curing activator (stearic acid and zinc oxide), antidegradation agent (wingstay-L), curing accelerator (DCBS), and curing agent (sulfur), by using a laboratory-sized internal mixer (Plastograph EC, Brabender GmbH & Co., Duisburg, Germany) at 40 ℃, a rotor speed of 40 rpm, and fill factor of 0.8. The recipe of the rubber compounds and the mixing times are displayed in Table 1.
The rubber compounds were compression-molded at 170 ℃ under the pressure of 900 psi following their respective curing times to obtain the crosslinked samples.
Characterizations. Transmission Electron Microscopy (TEM): The purified grafted copolymer latex was diluted and stained with osmium tetroxide (OsO4), prior to subjecting to TEM imaging. Transmission electron micrographs were recorded by using a JEOL JEM-2010 (JEOL Co., Japan) operating at 160 kV.
Fourier Transform Infrared Spectrometry (FTIR): Attenuated total reflectance Fourier-transform infrared (ATR-FTIR) spectra of all NR and purified MNR samples were recorded using a Spotlight 200i spectrometer (PerkinElmer Frontier, England). Each spectrum was recorded with 4 cm-1 resolution from 4000 cm-1 to 400 cm-1. The level of MA grafted onto NR was estimated from the absorbance ratio of IR peaks at 1720 cm-1 and 836 cm-1.2
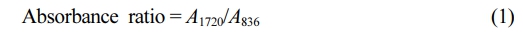
Curing Properties: The curing characteristics of NR and MNR compounds were tested by using a moving die rheometer, MDR 3000 Basic (Montech, Germany) at 170 ℃ for 20 min with oscillation degree and strain during curing of 0.5o and 7%, respectively, and a frequency of 1.67 Hz. The curing parameters, maximum torque (MH), torque difference (MH-ML), scorch time (ts1), curing time (t90), and cure rate index (CRI) were determined.
Thermomechanical Properties: The thermomechanical properties and crosslink densities of NR and the MNRs were measured using temperature scanning stress relaxation, performed with Brabender TSSR meter (Duisburg, Germany). All NR and MNR vulcanizates were placed in the heating chamber and stretched for 50% at 23 ℃. The samples were then pre-conditioned by keeping them under isothermal stress relaxation for 2 h. The short-time relaxation processes occurred during this time and were recorded. After 2 h, the specimens were heated at a rate of 2 ℃/min in a non-isothermal test, until the sample ruptured or the stress relaxation was completed. The relationships of force and temperature were finally obtained. The TSSR parameters, including the temperatures at which the force (F) had been reduced by 50% and 90% (T50 and T90) from its initial value (F0), and the apparent crosslink density (ν), were determined. The ν can be determined from the initial part of the stress vs. temperature curve by using the following equations.11
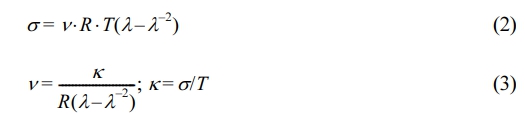
where R is the universal gas constant, l is the strain ratio, s is the mechanical stress, and T is the absolute temperature.
Tensile Properties: The dumbbell-shaped samples of crosslinked NR and MNRs were cut in accordance to ISO 37. The tensile properties were recorded by using a universal tensile testing device, LR5K Plus (Lloyd Instruments, UK). The test was performed at room temperature with a crosshead speed of 500 mm/min.
|
Table 1 Formulation of the NR and MNR Compounds |
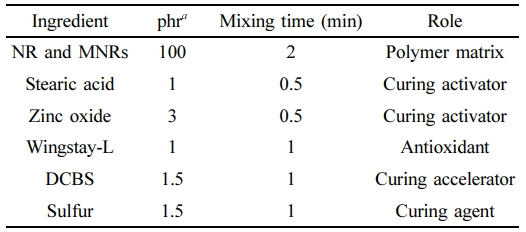
aphr = part(s) per hundred parts of rubber. |
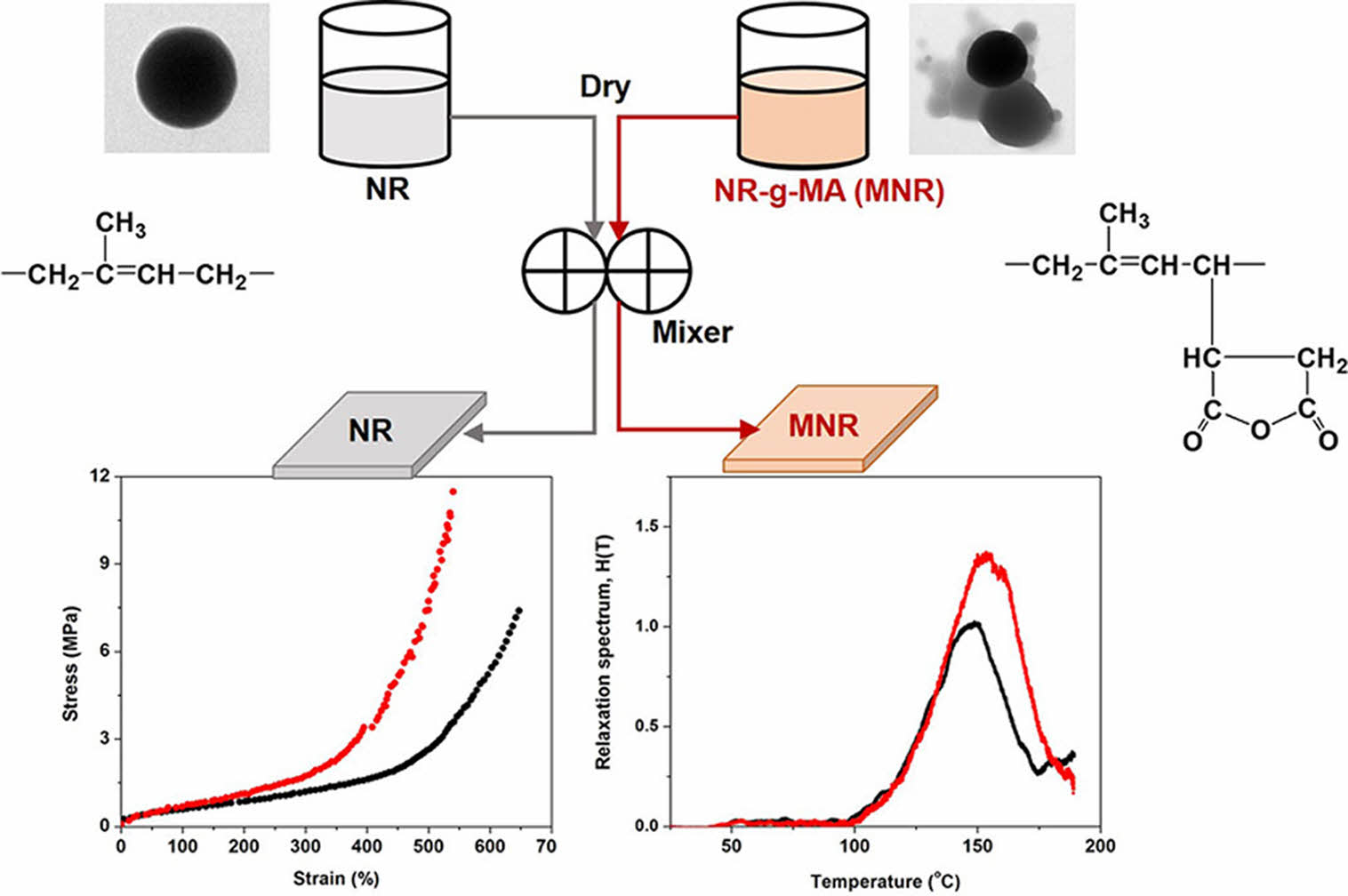
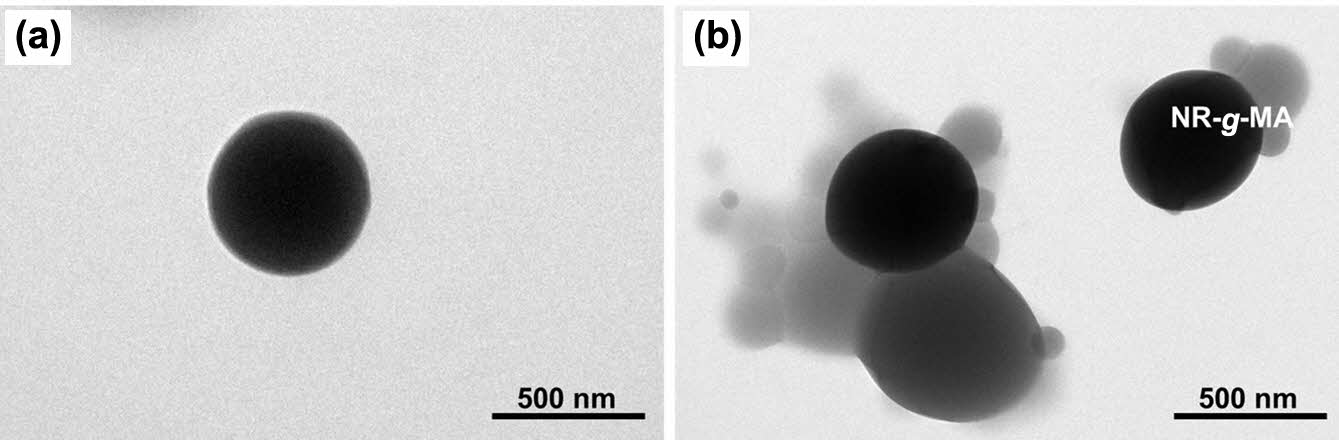
Figure 1 displays TEM images of neat NR latex particles and a representative of NR grafted with MA (containing 5 wt% MA). Prior to TEM imaging, both the NR latex and the resulting NR-MA were stained with OsO4 vapor to improve the phase contrast between NR and MA. It is believed that OsO4 can only react with the double bonds in NR, causing the NR particles to look dark in TEM micrographs.12 It is clearly seen from the images that the neat NR latex particles were near spherical in shape with smooth surfaces (Figure 1(a)), whereas the MA particles appeared brighter and globular (see Figure 1(b)). The NR latex particles grafted with 5 wt% MA had irregularly shaped morphologies with combination of both brighter globular MA and darker NR particles (Figure 1(b)). The many globular MA particles on the NR particle surface demonstrated that the grafting of MA onto the NR backbone was successful. It is also seen in Figure 1(b) that the amount of MA was irregularly attached to the NR particles. Some NR particles had many globular MA particles, whereas only few MAs were attached to others. This also indicates that the grafting of MA onto NR was random.
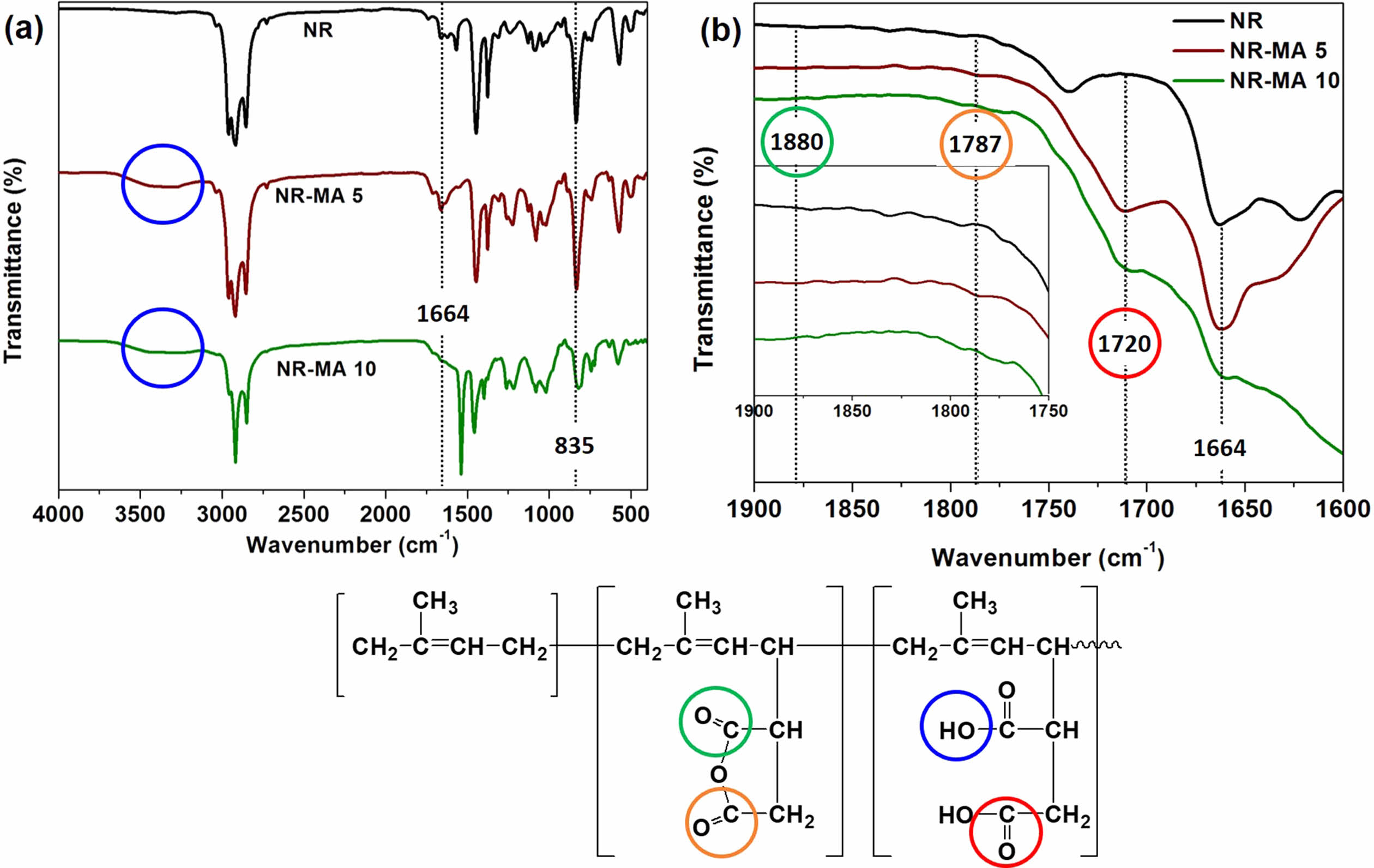
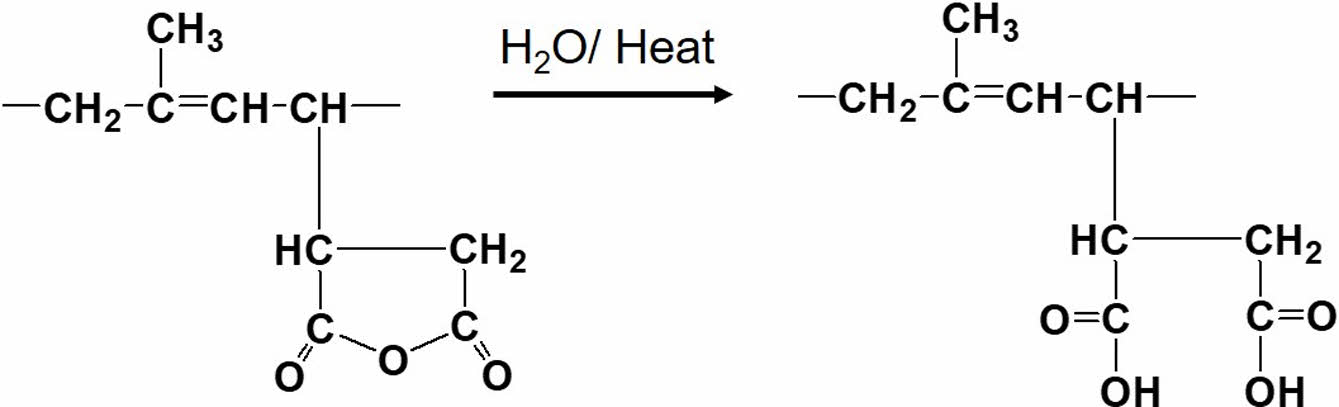
Figure 2 shows FTIR spectra of neat NR and purified MNR over the wavenumber ranges 4000-400 cm-1 (Figure 2(a)), and 1900-1600 cm-1 (Figure 2(b)). The FTIR spectra of the MNRs containing 5 wt% and 10 wt% MA were selected to represent the prepared MNRs. In Figure 2(a), the characteristic peaks of NR were at 1664 cm-1 and 835 cm-1, assigned to C=C stretching and C-H out-of-plane bending in the NR, respectively. 9 As compared to the neat NR, the NR-MA 5 and NR-MA 10 showed very small absorption bands at wavenumbers 1787 cm-1 and 1880 cm-1, assigned to the asymmetric and symmetric C=O stretching vibrations of succinic anhydride rings of cyclic anhydride.9,10,13 Also, bands at 1720 cm-1 and above 3000 cm-1 were noticed for the MNRs, probably due to carbonyl functional groups and -OH groups, respectively, stemming from the opening of ring structure of succinic anhydride, forming carboxylic acid in the MNR. It is well known that carboxylic acid in MNR is formed by the reaction of the anhydride functional group with moisture, according to the reaction shown in Figure 3.4,14
The TEM and FTIR results confirmed the existence of succinic anhydride groups grafted onto the NR. Apart from the cyclic anhydride forms, the MA grafted onto the NR possibly appeared in the ring-opening structures.
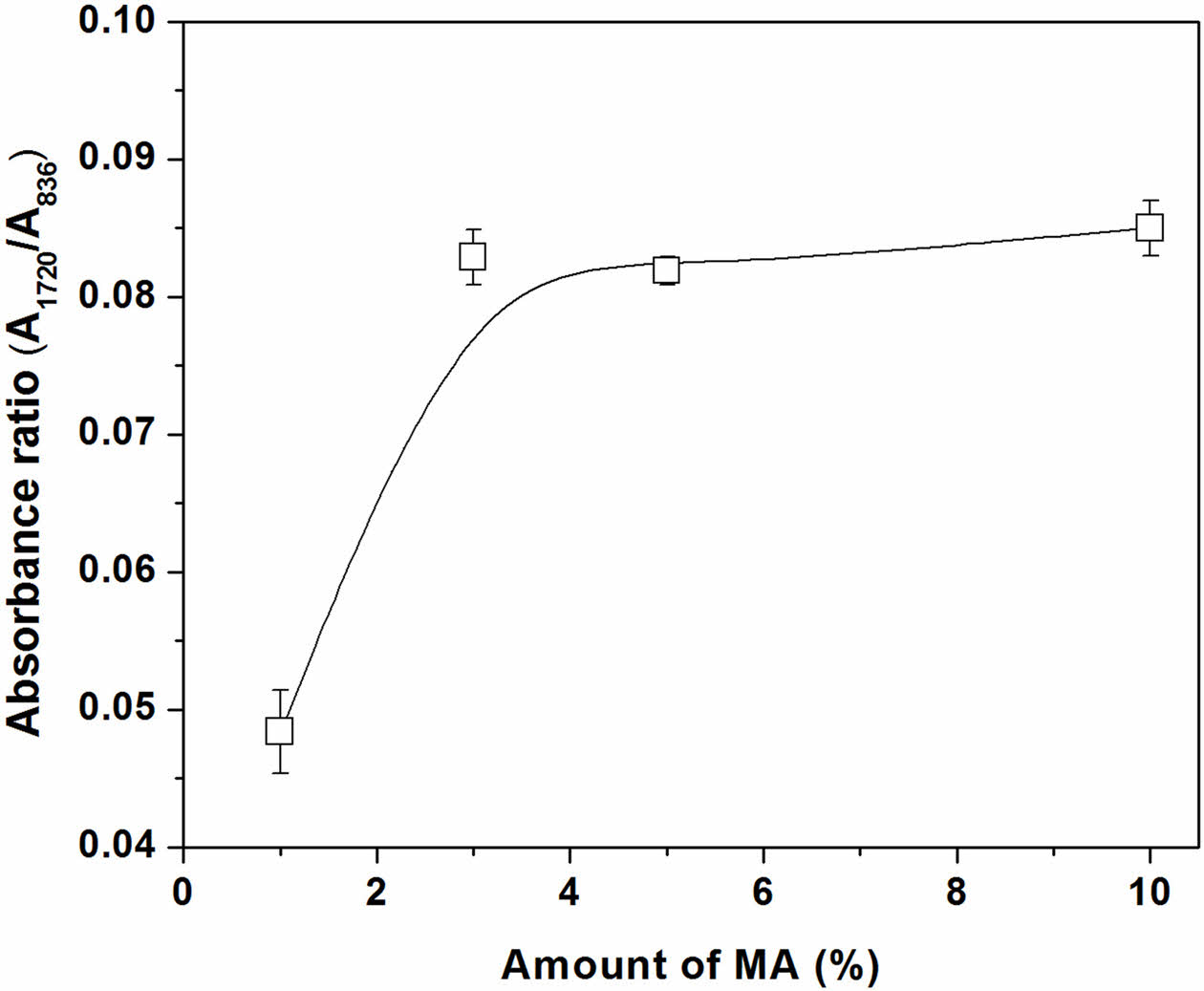
Figure 4 shows the level of grafted MA, which was estimated form the ratio of IR peaks at 1720 cm-1 to 836 cm-1 (A1720/A836). It is seen that absorbance ratio increased with MA content and reached its maximum when the MA content was 3 wt%. Further increase in the MA content no longer increased the absorbance ratio. This was probably due to the higher MA monomer concentration inducing side reactions, such as chain transfer to monomer, which competed with the grafting reactions and hence reduced the grafting efficiency.10 It should be noted here that the grating efficiency of MA onto NR latex was much lower than that of the one obtained with deproteinized NR.2 This is due to the fact that the protein in NR acts as a free-radical scavenger and terminates the active free-radical species participating in the grafting reactions.
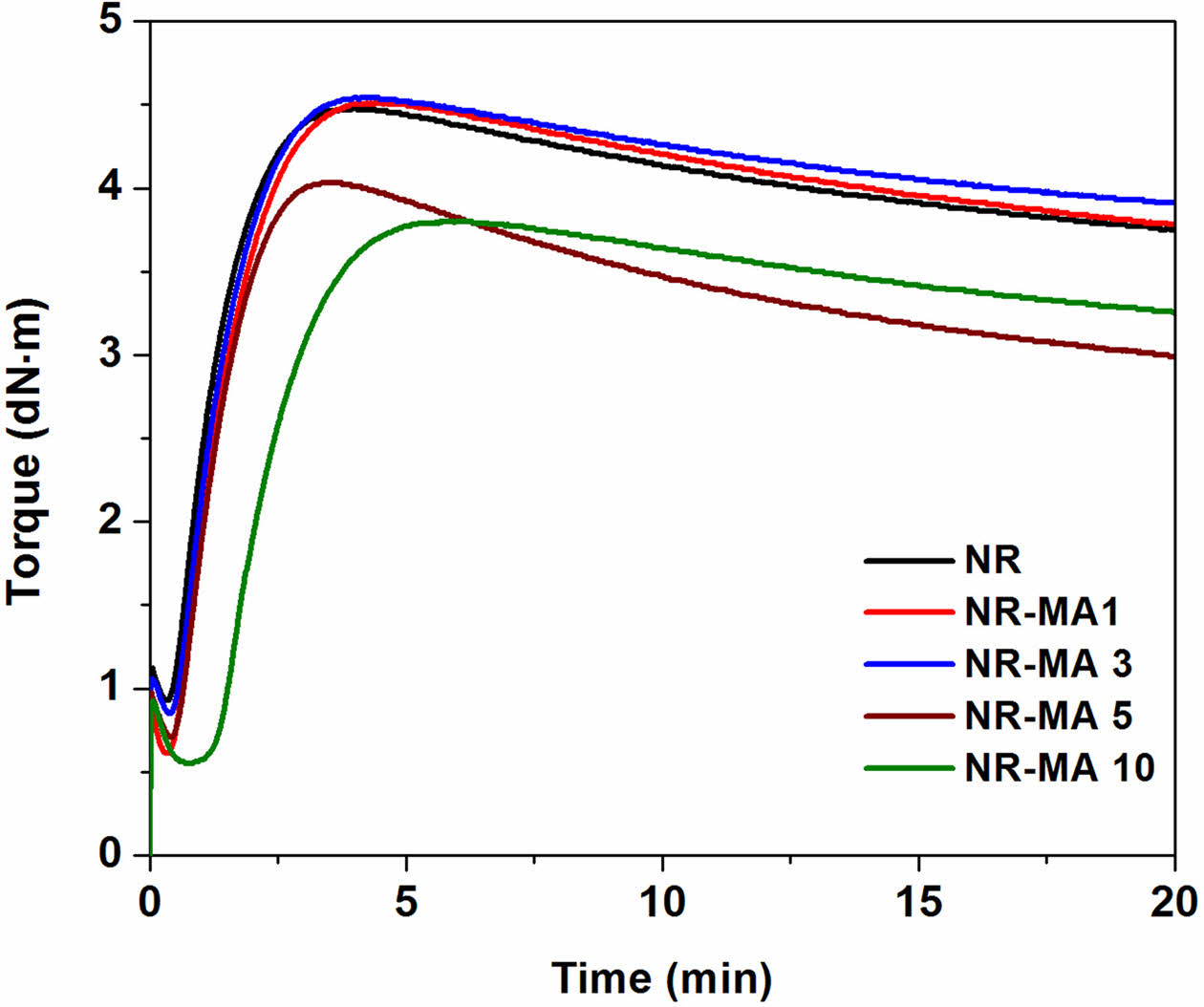
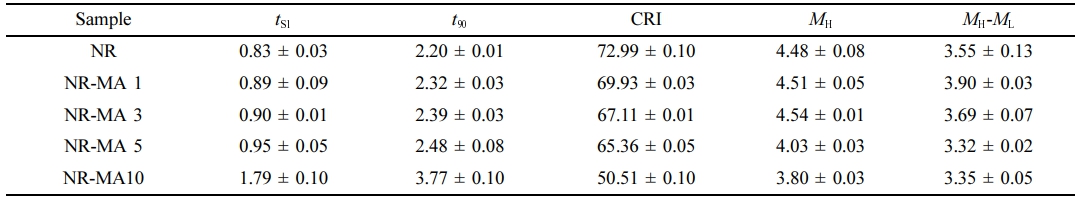
Figure 5 displays the curing characteristics of NR and the MNRs containing different amounts of MA and average values of the curing parameters scorch time (ts1), curing time (t90), cure rate index (CRI), maximum torque (MH), and torque difference (MH-ML) are summarized in Table 2.
As compared to the neat NR compound, ts1 and t90 of the MNRs increased with the MA content, meaning longer scorch and cure times. The acids from the ring opening of succinic anhydride groups delayed scorch and cure times in the MNRs.13 The delayed crosslinking reactions were confirmed by decreasing in CRI, proving that crosslinking reactions in the MNRs were retarded. Obviously, the maximum torque of all NR and MNR samples increased with time due to crosslinking induced by the sulfur crosslinking agent. In case of the MNRs, the maximum torque was found to increase with MA contents up to 3 wt%. A further increase of MA concentration reduced the maximum torque of MNRs. The increasing maximum torque indicates that the stiffness and crosslink density15 of NR increased with addition level of MA. These increases in both stiffness and crosslink density might be attributed to additional maleated linkages formed at an elevated temperature.4,13,15
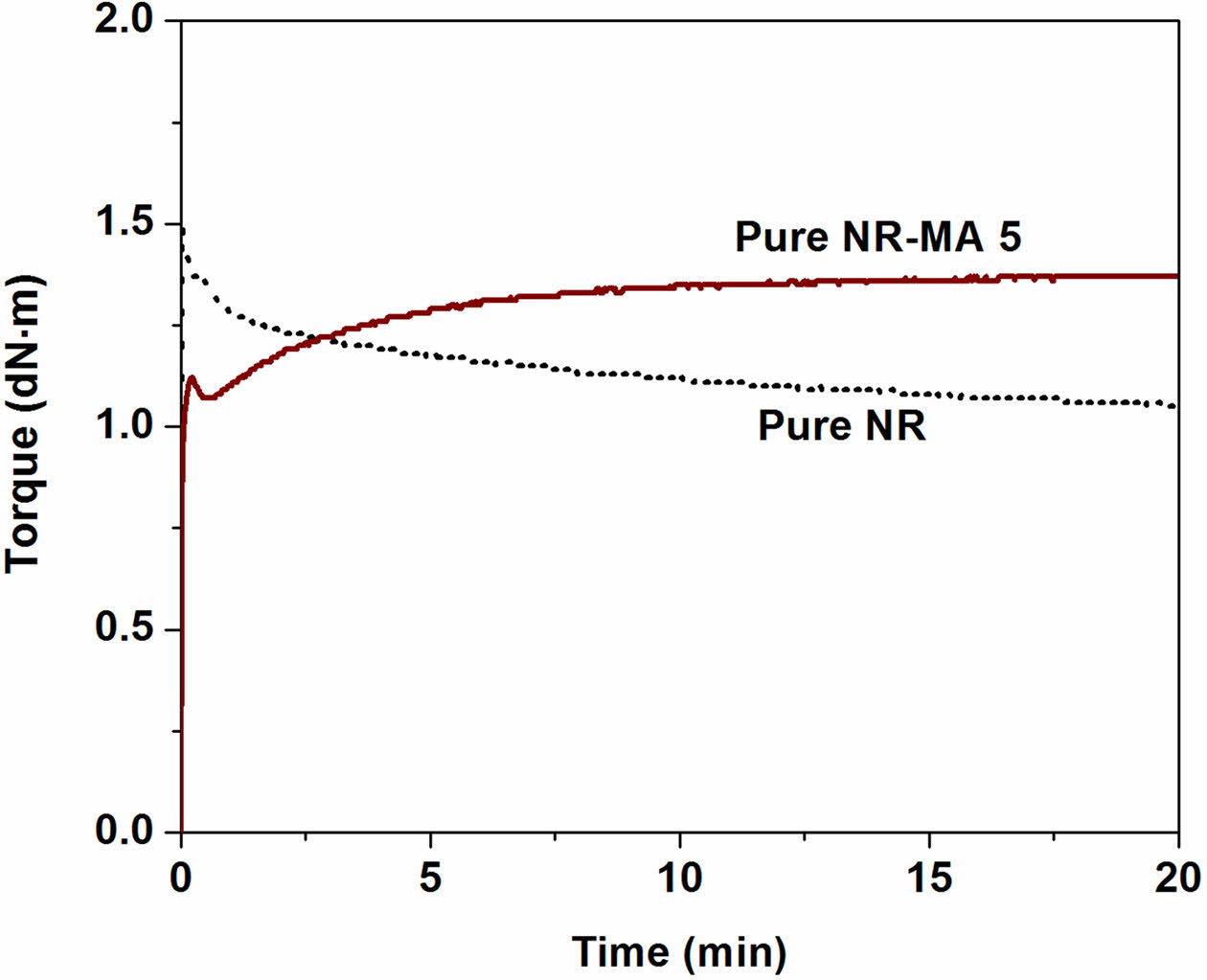
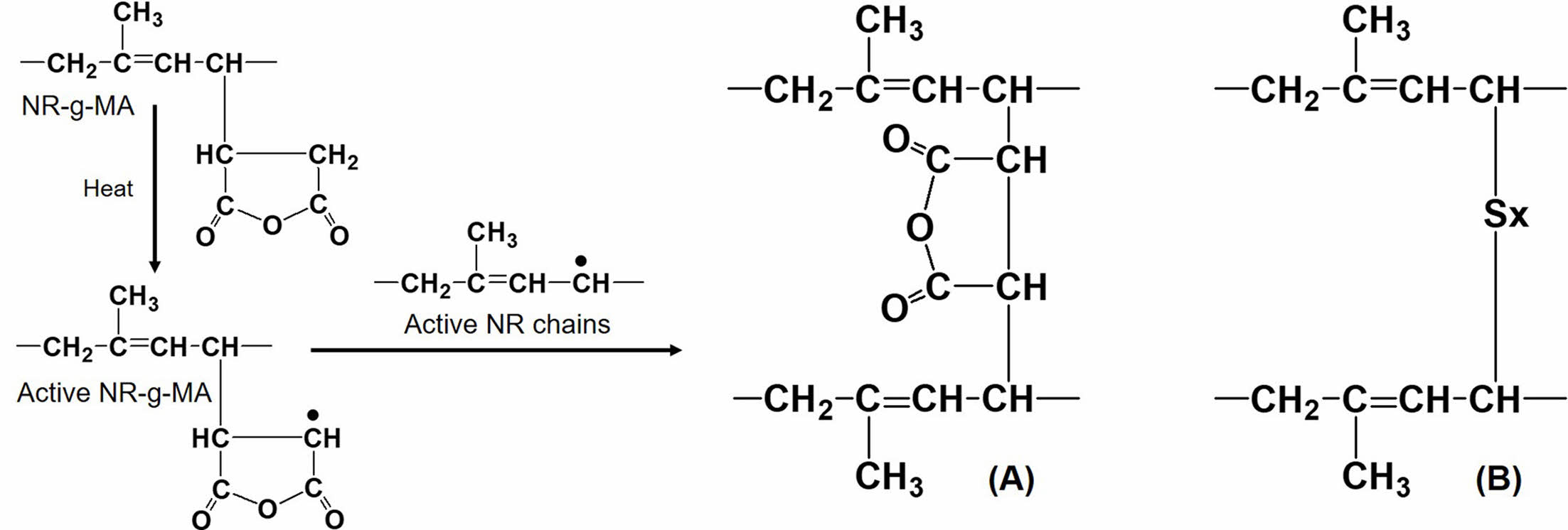
To confirm the presence of maleated linkages between NR molecules, the pure NR and the MNR containing 5 wt% MA (NR-MA 5) without addition of any chemical ingredients were subjected to rheometric testing at 170 ℃, and the results are shown in Figure 6. It is clear that the rheometric torque of pure NR-MA 5 was developed during vulcanization at an elevated temperature, whereas the torque of pure NR was not increasing. This evidence confirms the self-crosslinking reactions between MA groups grafted onto NR, forming maleated linkages among MNR chains during vulcanization. It is assumed that the active sites on MNR generated by heat during vulcanization would react with active NR chains as shown in Figure 7, forming maleated linkages (Figure 7(a)). The maleated linkages contributed as additional crosslinks to the conventional sulfur linkages (Figure 7(b)), resulting in an increased stiffness and crosslink density in the MNRs.
However, the greatest MH-ML was for NR-MA 1, implying that the highest crosslink density was obtained when the MA content was 1 wt%. Further increases in the MA level decreased the crosslink density, probably due to the formation of MA-particle aggregates. These aggregates may reduce the efficiency of maleated linkage formation between rubber molecules, and may also act as plasticizer due to the low molecular weight of MA, promoting the ease of molecular motion. Another possible reason for reducing MH-ML is that the higher MA addition level increased the acidity of the MNR, reducing the extent of crosslinking reactions as previously seen from the CRI decreasing.
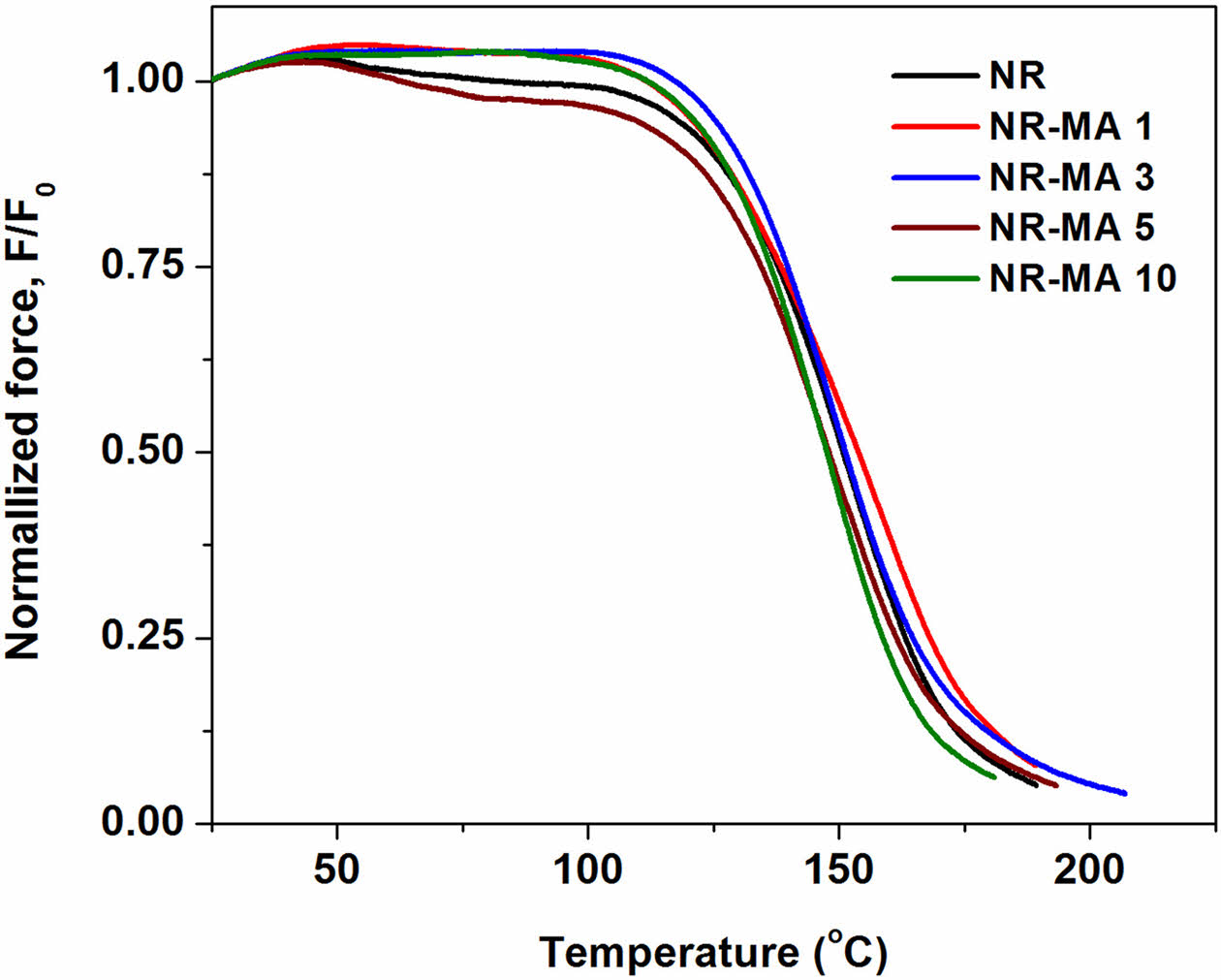
Figure 8 shows normalized force curves for the MNR vulcanizates with different MA contents during non-isothermal testing. For comparison, the normalized force curve of pure NR vulcanizate was also included. It is well known that a slight increase of initial normalized force at temperatures of 30-50 ℃ was due to the entropy effect,16 seen in all cases. The disengagement of physical bonding among rubber molecules caused a slight reduction of force over 50-100 ℃,17,18 and the thermo-oxidative chain scission and cleavage of network bridges between rubber molecules resulted in an abrupt decrease of force to zero at temperatures over 100 ℃.19
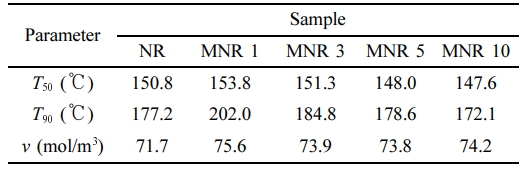
It is also seen that the normalized force curves of MNRs were above that of the NR in all tested temperatures, except for NR-MA 5 and NR-MA 10. As compared to the NR, the increased normalized force curves for NR-MA 1 and NR-MA 3 revealed that the stability of NR chains to elevated temperature was improved. The formation of maleated bonds provides greater resistance to an elevated temperature due to the higher bonding energy of carbon-carbon bonds (80 kcal/mol) than that of sulfide (-Sx-) bond (34 kcal/mol).20 In contrast, a reduction of the normalized force curves for NR-MA 5 and NR-MA 10 was attributed to the decrease in crosslink density, as earlier suggested by rheometry results (Figure 5 and Table 2). The TSSR parameters obtained from plot of force versus temperature, including T50, T90, and ν, are summarized in Table 3. It is well accepted that the T50 is associated with the upper limit of service temperature range, whereas T90 is related to material stability.21,22 From Table 3, it can be seen that the T50 and T90 of NR were increased by a low level of MA grafting, i.e., at 1 and 3 wt%, due to the formation of maleated linkages. A higher level of MA reduced T50 and T90 due to the excess of MA that may act as a plasticizer, facilitating the movement of rubber chains under test conditions. A drastic improvement in both T50 and T90 was seen for NR-MA 1, implying that the highest service temperature and thermo-stability were achieved at 1 wt% MA content, among the levels tested. Considering the ν of various samples, the ν of vulcanized NR originated from conventional sulfur agent was about 71.7 mol/m³, whereas the MNRs showed higher values of ν ranging within 73.8-75.6 mol/m3. This supports the concept of additional maleated linkages formed between MNR molecules. However, the largest ν was found for the case NR-MA 1 (75.6 mol/m3), having also the greatest service temperature and the best material stability. Increasing the MA concentration beyond 1 wt% reduced the crosslink density due to the formation of MA-particle aggregates and the increase of acidity in the MNR, limiting the extent of crosslinking reactions.
Unlike other properties, the trend of ν obtained from TSSR test was slightly differed from others. This discrepancy was probably due to the difference of testing condition, e.g., the samples were stretched for 50% and hold for 2 h prior to crosslink density test for TSSR whereas other tests were not.
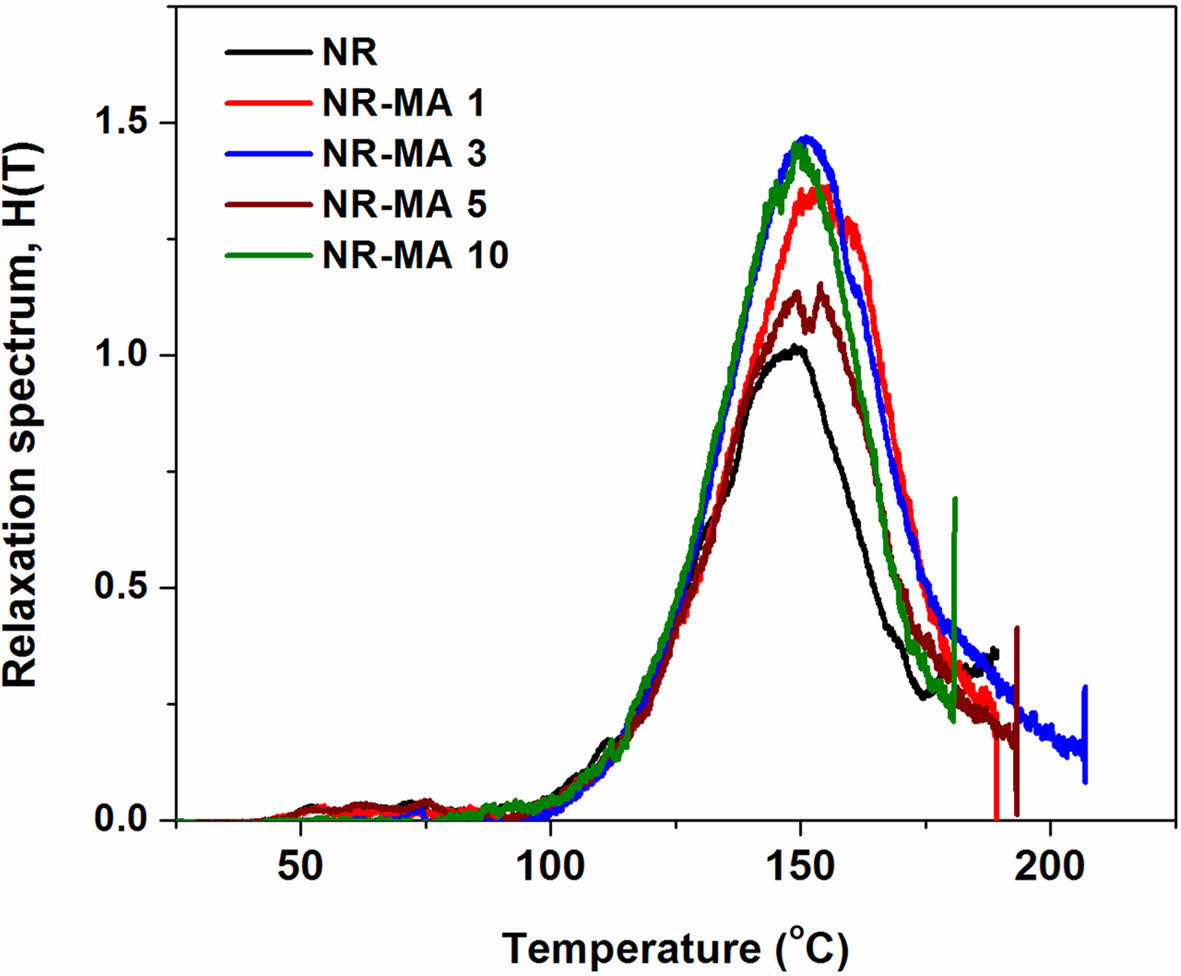
Figure 9 shows the relaxation spectra of vulcanized pure NR and the MNRs as functions of temperature. It has been reported that the relaxation of rubber chains after cleaving physical interactions can be observed from the peak in the lower temperature range, while the higher temperatures relate to chemical bonding.19 Thus, the small peaks observed at 50-100 ℃ in all cases corresponded to the relaxation of rubber chains after the detachment of their physical bonds, while the peak at about 148 ℃ corresponded to the relaxation of rubber molecules when sulfur linkages were degraded.23 Due to the C-C bonds by maleated linkages, the relaxation peak observed in MNRs moved toward higher temperatures. A significant shift in the relaxation peak was observed for NR-MA 1, which was roughly at 10 ℃ above that of the vulcanized NR. This suggests that the best balance of network structure in the rubber was obtained in the case of NR-MA 1.
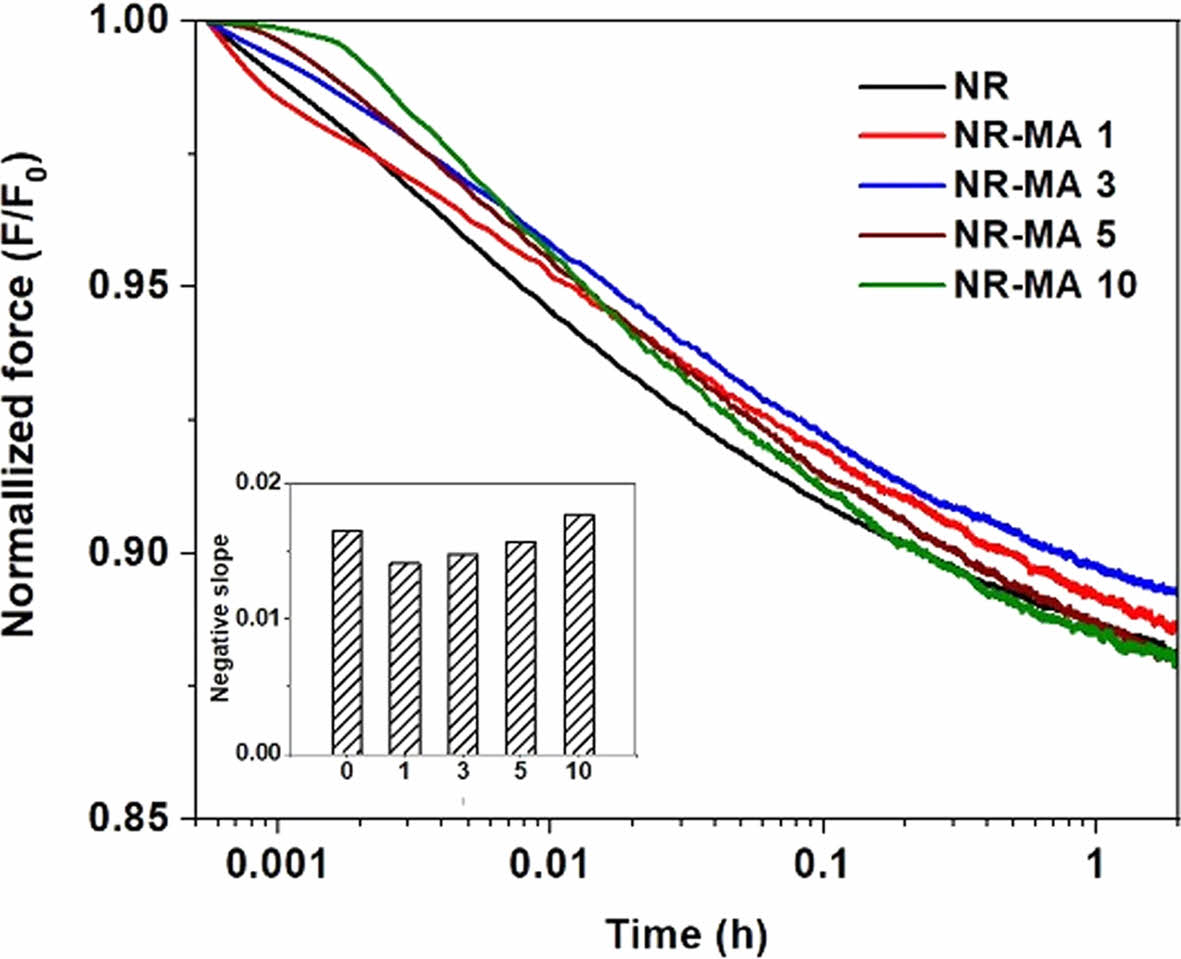
Figure 10 shows normalized force (F/F0) curves for vulcanized NR and the MNRs during isothermal stress relaxation. It is seen that all NR and MNR samples showed a linear decrease of stress over time.
Interestingly, the rate of stress relaxation was found to decrease with the grafting level of MA onto the NR. The magnitude of the negative slope in the insert within Figure 10 was smallest for the NR-MA 1, implying that the molecular relaxation was comparatively slow in this sample. The molecular relaxation rate was found to increase again when the MA concentration was greater than 1 wt%. The slowest stress relaxation obtained in the NR-MA 1 could be explained by the highest crosslink density of this case. The highest crosslink density provided the most tie points between rubber chains, preventing the polymer chains from sliding by each other and restricting their mobility. On the other hand, a reduction of crosslink density would increase the relaxation rate due to the easier movement of rubber chains when a load is applied. Thus, the rate of relaxation increased again after 1 wt% of MA. Since the greatest service temperature, thermo-stability of material and slowest molecular relaxation were found for the NR-MA 1, this loading level may be the best option for the latex technique that attaches MA onto NR.
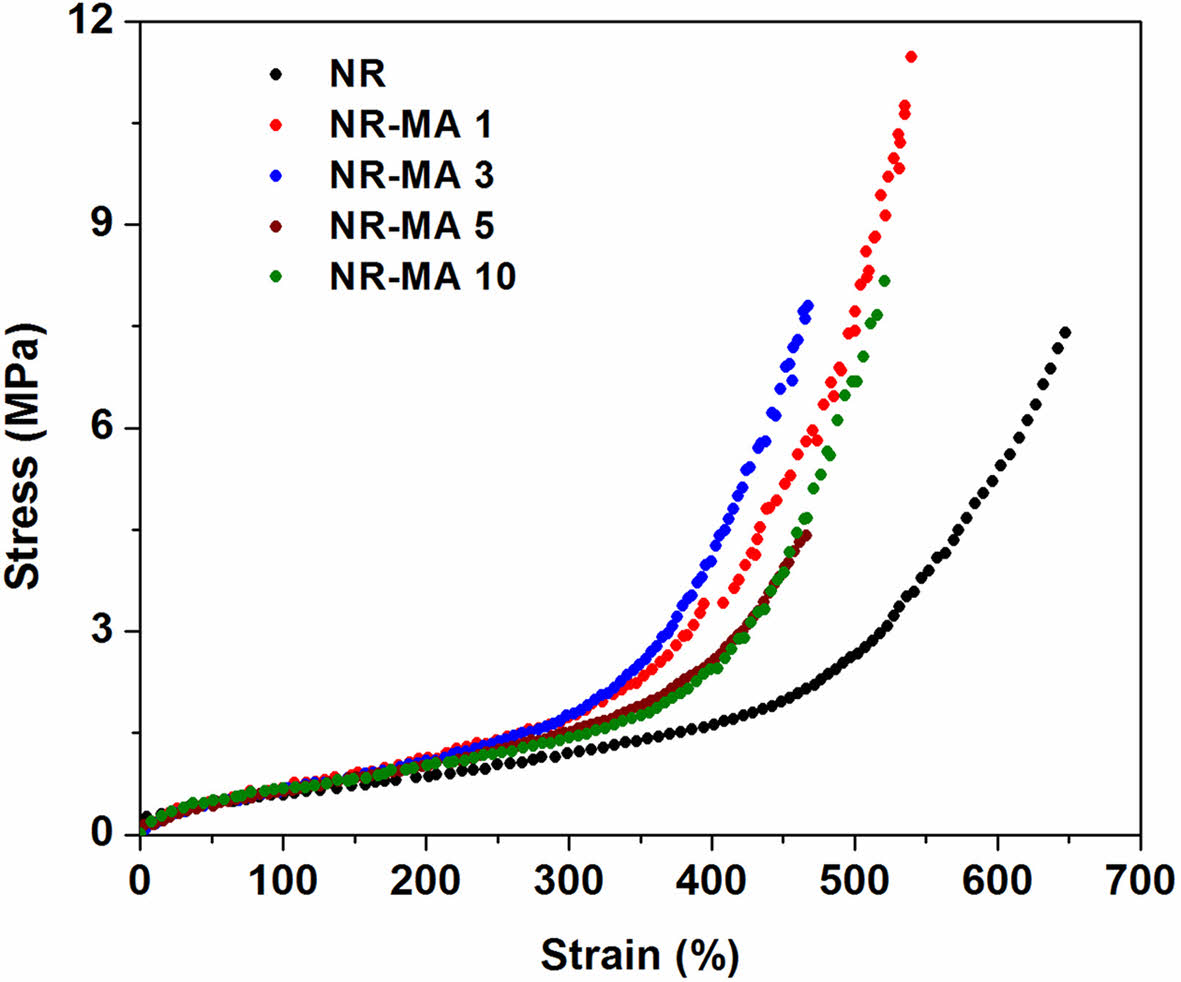
Figure 11 shows representative stress-strain curves for NR and the MNR as vulcanizates. Apparently, the characteristic of stress and strain of the MNR vulcanizates depended mainly on the level of MA. The tensile properties, 100% and 300% moduli, tensile strength, and elongation at break, are summarized in Table 4. The 100% and 300% moduli and tensile strength increased with MA content, due to increasing crosslink density resulting from additional maleated linkages. The increased crosslinking level provides more joining points between the molecular chains, increasing the stiffness of the rubber. As a result of crosslink density increase, the elongation at break of the MNRs was decreasing with MA content. The maximum tensile strength was achieved by NR-MA 1 with a 53% improvement over the vulcanized NR. Further increase in the MA level reduced the tensile strength due to the reduction of total crosslink density and/or the decreased grafting efficiency of MA onto the NR molecules.
Based on the results obtained in this study, the crosslink density of MNR vulcanizates was greater than that of the vulcanized pure NR due to additional maleated linkages formed in the MNRs. These additional linkages enhanced the thermo-stability and mechanical properties of the NR. The greatest thermomechanical and tensile properties were achieved by NR-MA 1 among the cases tested, also having the highest crosslink density. This might be a near optimal content level for grafting MA onto the NR chains via the latex technique.
|
Figure 1 TEM images of (a) pure NR; (b) NR-MA 5 (or MNR) particles. |
|
Figure 2 FTIR spectra of neat NR, and MNR containing 5% and 10% of MA in range of (a) 4000-400 cm-1; and (b) 1900-1600 cm-1. |
|
Figure 3 Possible ring opening reaction of the anhydride functional group in MNR. |
|
Figure 4 Absorbance ratio (A1720/A836) of MNRs with varying amounts of MA. |
|
Figure 5 Time profiles of torque during curing of NR and the MNR compounds. |
|
Figure 6 Curing curves of pure NR and the MNR containing 5 wt% of MA without addition of rubber additives. |
|
Figure 7 Reactions between rubber chains: (a) maleated linkages in MNR; (b) sulfur linkages in NR |
|
Figure 8 Normalized force curves from isothermal stress relaxation for NR and the MNR vulcanizates. |
|
Figure 9 Relaxation spectra of NR and the MNR vulcanizates during isothermal stress relaxation. |
|
Figure 10 Normalized force (F/F0) during isothermal stress relaxation for NR and the MNRs. |
|
Figure 11 Stress-strain curves for MNRs with various MA contents. |
|
Table 3 TSSR Parameters (T50, T90, and ν) for Vulcanized Samples of NR and the MNR Vulcanizates |
|
Table 4 Tensile Properties of NR and MNR Vulcanizates |
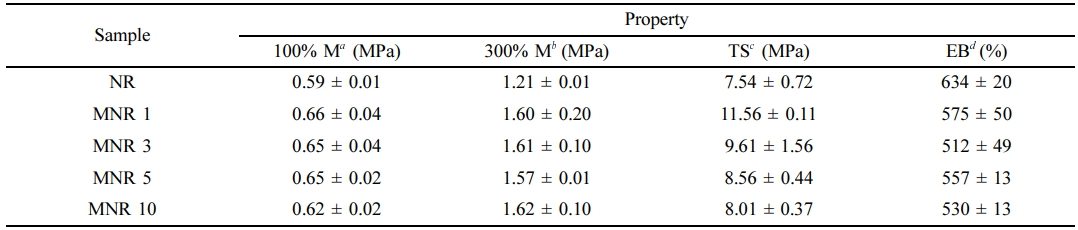
a100%M = 100% Modulus. b300%M = 300% Modulus. |
Effects of MA content on properties of MNRs were investigated. The MNRs with various MA contents were firstly prepared by grafting polymerization of MA onto NR via latex technique. The TEM imaging and FTIR spectrometry confirmed successful grafting. Rheometric testing revealed that the scorch time and cure time of MNRs were longer than that of the NR, due to the acidity of MA retarding the crosslinking reactions and/or the decreased grafting efficiency of MA onto NR. The maximum torque and torque difference were also improved over the NR due to the maleated linkages formed among MNR molecules. The additional maleated linkages induced a greater crosslink density and thermo-stability in the MNRs, as demonstrated by the TSSR test. The tensile properties of NR were also improved by grafting MA onto the NR. A drastic enhancement of thermo-stability by 10 ℃ and of tensile strength by 53% over NR were achieved when the MNR had a 1 wt% MA level. Further increase in the MA concentration reduced the thermo-mechanical properties and tensile strength, with a decreased crosslink density.
- 1. Phinyocheep, P. Chemical Modification of Natural Rubber (NR) for Improved Performance. In Chemistry, Manufacture and Applications of Natural Rubber; Kohjiya, S., Ikeda, Y., Eds.; Woodhead Publishing: Cambridge, 2014; pp 68-118.
-

- 2. Wongthong, P.; Nakason, C.; Pan, Q.; Rempel, G. L.; Kiatkamjornwong, S. Modification of Deproteinized Natural Rubber via Grafting Polymerization with Maleic Anhydride. Eur. Polym. J. 2013, 49, 4035-4046.
-

- 3. Nakason, C.; Saiwari, S.; Kaesaman, A. Rheological Properties of Maleated Natural Rubber/Polypropylene Blends with Phenolic Modified Polypropylene and Polypropylene-g-Maleic Anhydride Compatibilizers. Polym. Test. 2006, 25, 413-423.
-

- 4. Nakason, C.; Saiwaree, S.; Tatun, S.; Kaesaman, A. Rheological, Thermal and Morphological Properties of Maleated Natural Rubber and Its Reactive Blending with Poly(methyl methacrylate). Polym. Test. 2006, 25, 656-667.
-

- 5. Nakason, C.; Kaesaman, A.; Samoh, Z.; Homsin, S.; Kiatkamjornwong, S. Rheological Properties of Maleated Natural Rubber and Natural Rubber Blends. Polym. Test. 2002, 21, 449-455.
-

- 6. Ismail, H.; Rusli, A.; Rashid, A. A. Maleated Natural Rubber as a Coupling Agent for Paper Sludge Filled Natural Rubber Composites. Polym. Test. 2005, 24, 856-862.
-

- 7. Brydson, J. A. Rubber Chemistry; Applied Science Publishers: London, 1978.
- 8. Nakason, C.; Kaesaman, A.; Samoh, Z.; Homsin, S.; Kiatkamjornwong, S. Rheological and Curing Behavior of Reactive Blending, I. Maleated Natural Rubber-Cassava Starch. J. App. Polym. Sci. 2001, 81, 2803-2813.
-

- 9. Hayeemasae, N.; Sensem, Z.; Sahakaro, K.; Ismail, H. Maleated Natural Rubber/Halloysite Nanotubes Composites. Processes 2020, 8, 286.
-

- 10. Nakason, C.; Kaesaman, A.; Supasanthitikul, P. The Grafting of Maleic Anhydride onto Natural Rubber. Polym. Test. 2004, 23, 35-41.
-

- 11. Vennemann, N.; Bokamp, K.; Broker, D. Crosslink Density of Peroxide Cured TPV. Macromol. Symp. 2006, 245-246, 641-650.
-

- 12. Kangwansupamonkon, W.; Gilbert, R. G.; Kiatkamjornwong, S. Modification of Natural Rubber by Grafting with Hydrophilic Vinyl Monomers. Macromol. Chem. Phys. 2005, 206, 2450-2460.
-

- 13. Sahakaro, K.; Beraheng, S. Reinforcement of Maleated Natural Rubber by Precipitated Silica. J. Appl. Polym. Sci. 2008, 109, 3839-3848.
-

- 14. Ichazo, M. N.; Albano, C.; Hernandez, M.; Gonzalez, J.; Pena, J. Characterization of Natural Rubber/Cassava Starch/Maleated Natural Rubber Formulations. Rev. Latinoam. Metal. Mater. 2011, 31, 71-84.
- 15. Masa, A.; Soontaranon, S.; Hayeemasae, N. Influence of Sulfur/Accelerator Ratio on Tensile Properties and Structural Inhomogeneity of Natural Rubber. Polym. Korea 2020, 44, 519-526.
-

- 16. Vennemann, N.; Wu, M. Thermoelastic Properties and Relaxation Behavior of S-SBR/Silica Vulcanizates. Rubber World 2012, 246, 18-23.
- 17. Hayeemasae, N.; Soontaranon, S.; Masa, A. Influence of Different Vulcanizing Agents on Structures and Properties of Sepiolite-Filled Natural Rubber Composites. Express Polym. Lett. 2023, 17, 181-195.
-

- 18. Promsung, R.; Nakaramontri, Y.; Uthaipan, N.; Kummerlowe, C.; Johns, J.; Vennemann, N.; Kalkornsurapranee, E. Effects of Protein Contents in Different Natural Rubber Latex Forms on the Properties of Natural Rubber Vulcanized with Glutaraldehyde. Express Polym. Lett. 2021, 15, 308-318.
-

- 19. Srinivasan, N.; Bokamp, K.; Vennemann, N. New test method for the characterisation of filled elastomers. Kautsch. Gummi Kunstst. 2005, 58, 650-655.
- 20. Rodgers, B. Rubber Compounding: Chemistry and Applications; CRC Press: New York, 2015.
- 21. Pongdong, W.; Kummerlowe, C.; Vennemann, N.; Thitithammawong, A.; Nakason, C. A Comparative Investigation of Rice Husk Ash and Siliceous Earth as Reinforcing Fillers in Dynamically Cured Blends of Epoxidized Natural Rubber (ENR) and Thermoplastic Polyurethane (TPU). J. Polym. Environ. 2018, 26, 1145-1159.
-

- 22. Chatterjee, T.; Vennemann, N.; Naskar, K. Temperature Scanning Stress Relaxation Measurements: A Unique Perspective for Evaluation of the Thermomechanical Behavior of Shape Memory Polymer Blends. J. Appl. Polym. Sci. 2018, 135, 45680.
-

- 23. Matchawet, S.; Kaesaman, A.; Vennemann, N.; Kumerlowe, C.; Nakason, C. Effects of Imidazolium Ionic Liquid on Cure Characteristics, Electrical Conductivity and Other Related Properties of Epoxidized Natural Rubber Vulcanizates. Eur. Polym. J. 2017, 87, 344-359.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2022 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2023; 47(5): 565-573
Published online Sep 25, 2023
- 10.7317/pk.2023.47.5.565
- Received on Jan 25, 2023
- Revised on Jul 6, 2023
- Accepted on Jul 12, 2023
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
- Conflict of Interest
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Abdulhakim Masa
-
**Research Unit of Advanced Elastomeric Materials and Innovations for BCG Economy (AEMI), Faculty of Science and Technology, Prince of Songkla University, Pattani Campus, Pattani, 94000, Thailand
****Rubber Engineering & Technology Program, International College, Prince of Songkla University, Hat Yai, Songkhla, 90110, Thailand - E-mail: abdulhakim.m@psu.ac.th
- ORCID:
0000-0002-0577-4844









 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.