- Characterization and Investigation of Electrical Properties of Epoxidized Soybean Oil-tri Salicyl Borate Polymer
Gökhan Çayli†
 , Hüseyin Esen*, Demet Gürbüz**, Cengiz Polat Uzunoğlu***, Adem Çinarli**, and Cengiz Kahraman
, Hüseyin Esen*, Demet Gürbüz**, Cengiz Polat Uzunoğlu***, Adem Çinarli**, and Cengiz KahramanIstanbul University-Cerrahpaşa, Engineering Faculty, Department of Engineering Sciences, Istanbul 34320, Turkey
*Kocaeli University, Kocaeli Vocational School, Chemistry and Chemical Processing Technologies, Chemical Technology Program, Kocaeli 41140, Turkey
**Istanbul University-Cerrahpaşa, Engineering Faculty, Department of Chemistry, Istanbul 34320, Turkey
***Istanbul University-Cerrahpaşa, Engineering Faculty, Department of Electrical Engineering, Istanbul 34320, Turkey- 에폭시화된 대두유와 Trisalicyl Borate 고분자의 전기적 특성 분석 및 연구
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
In this study, polymerization and characterization of trisalicyl borate (TSB) with epoxidized soybean oil (ESO) was evaluated. Synthesis of TSB is involved by the reaction between boric acid and salicylic acid at the first step of the reaction. Then TSB was polymerized with ESO at 95 ℃ for 6 hours. All the compounds synthesized were characterized by FTIR and 1H NMR techniques. Characteristic bands of the boron esters appeared at 1644, 1610, 1578, 1472, 1276, 784, 757, 656, and 530 cm-1. 1H NMR technique indicated that hydrogens of the free carboxyl groups revealed at 10.8 ppm. The resultant polymers of TSB and ESO were rubbery materials. Mechanical, thermal and electrical properties of the TSB-ESO polymer was determined. They exhibit small stress at break values with high strain percentages. Thermal and mechanical characterization of the polymers were performed by differential scanning calorimetry (DSC), thermogravimetric analysis (TGA) and Stress-Strain tests. It was found that TSB-ESO polymer exhibited highest swelling ratio in toluene. The TSB-ESO polymer was a rubbery material that showed 0.1 MPa stress at break value with 240% elongation. Thermal properties of the synthesized polymer were evaluated with DSC and TGA methods. TSB-ESA showed one Tg at -2 ℃ and a melting peak at 92 ℃. TGA plot showed 2 segmental weight loss. As an indicator for thermal stability, 5% weight loss temperature was measured as 112 ℃. Additionally, DC analysis reveals that the resistivity and conductivity of the polymeric sample is measured as 2.375 MΩm and 0.401 µS/m respectively.
Trisalicyl borate and epoxidized soybean oil polymer is a rubbery material. Like a rubbery material, this polymer show low tensile strength with high strain. This polymer have good insulation properties and it would be used for electrical applications where high tensile strength is not needed such as transformer insulator or wire insulator.
Keywords: plant oils, epoxidized soybean oil, salicylic acid, boric acid esters, renewable resources.
This work was supported by Scientific Research Projects Coordination Unit of Istanbul University-Cerrahpaşa. Project number 23154.
The authors declare that there is no conflict of interest.
Human settlements are at the center of sustainable development, and demographic data contains important implications for the economy, social development, and environmental sustainability.1 It is understood that the population, which was 2.5 billion in 1950, reached 6.1 billion by the year 2000 (in a 50-year period), with a nearly double increase. In the future projection, it is foreseen that the world population will increase by 100% after only 80 years, and a population of 11.2 billion will be formed in 2100.2 As the population increases, the variety of materials used in technology also increases. However, these materials are expected to be compatible with non-sustainable development goals and the environment. The variety of petroleum based materials utilized in industrial products is expanding along with the world population. Due to the sustainable development and environmental concerns, industrial designs should use new materials that obtained from renewable resources.
The development of alternative sources is an important issue both in terms of sustainability and minimizing the environmental impact. Additionally, these alternative resources would provide a great advantage in maximum adaptation to innovative technologies. Therefore, the demand for innovative materials is increasing day by day. Plant oil derived materials are getting more and more attention todays because they would be promising materials for environment, sustainable development and innovative technologies.
Plant oil triglycerides are valuable materials due to their structure. Complex nature of those molecules makes them valuable starting material for many compounds and polymers.3-4 Additionally, plant oil triglycerides have positive impacts on the environment and sustainable development.5-6 A typical plant oil triglyceride contains 4 different reactive positions. Those are ester group, α-carbon to carbonyl, double bonds, allylic and double allylic positions respectively.7-9 Conversion of those positions via special reactions lead to triglycerides based reactive monomers and polymers.10-12
Addition to double bonds is one of the most preferred method to modify plant oil triglycerides. Halogen and hydrogen halide additions are extensively preferred. Besides this type of addition reaction, insertion of oxygen atom to double bonds would yield epoxidized plant oil triglycerides. Those compounds are industrially accessible. Epoxidized soybean oil (ESO) is one of the most produced epoxidized plant oil triglycerides.
Epoxide groups are oxygen bearing three membered strained rings. Thus, they are reactive to nucleophilic and electrophilic attacks. When an epoxide ring is reacted with a carboxylic acid. The product is a β-hyroxy ester. Polymerization reaction of ESO with dicarboxylic acids are studied well.13-15
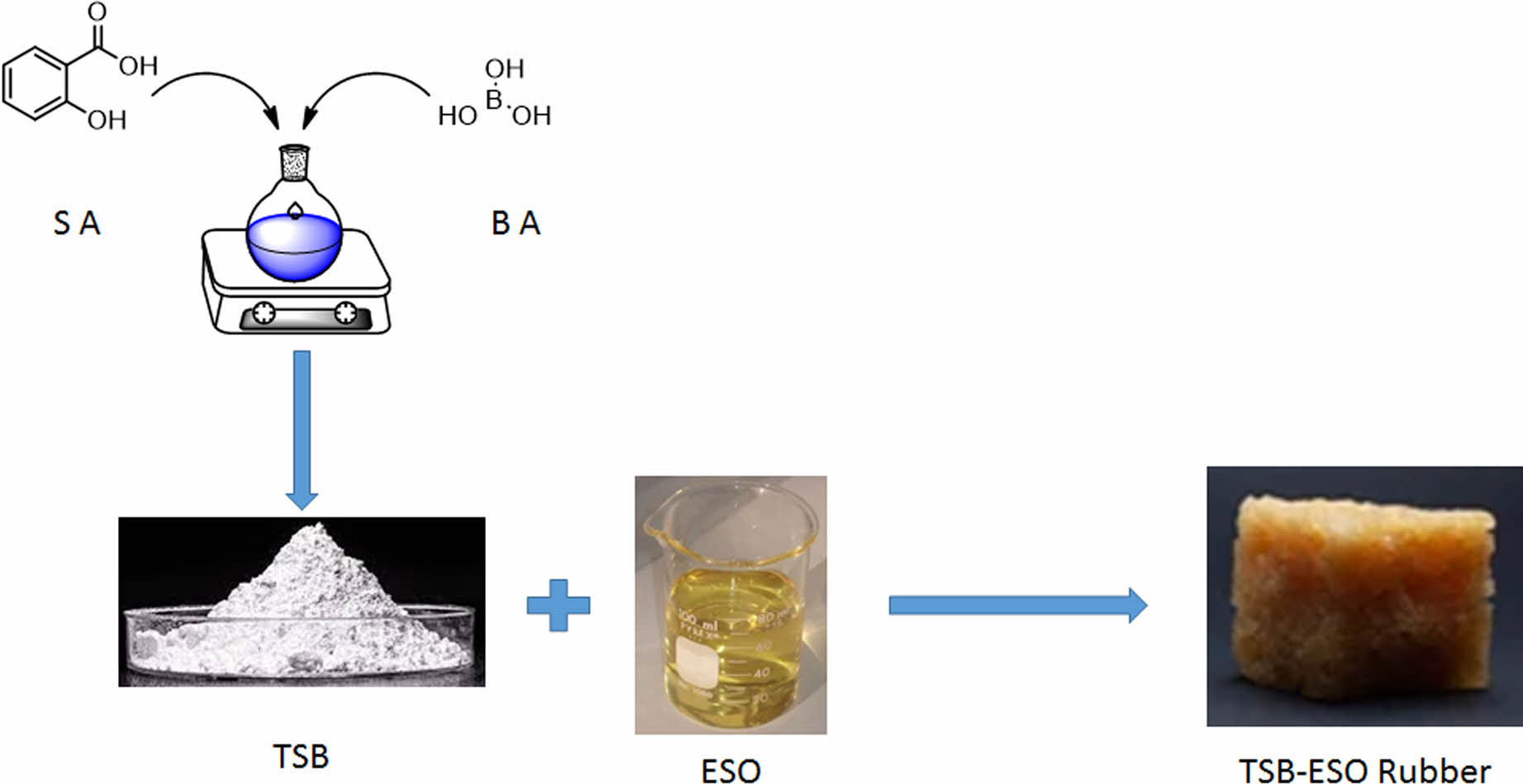
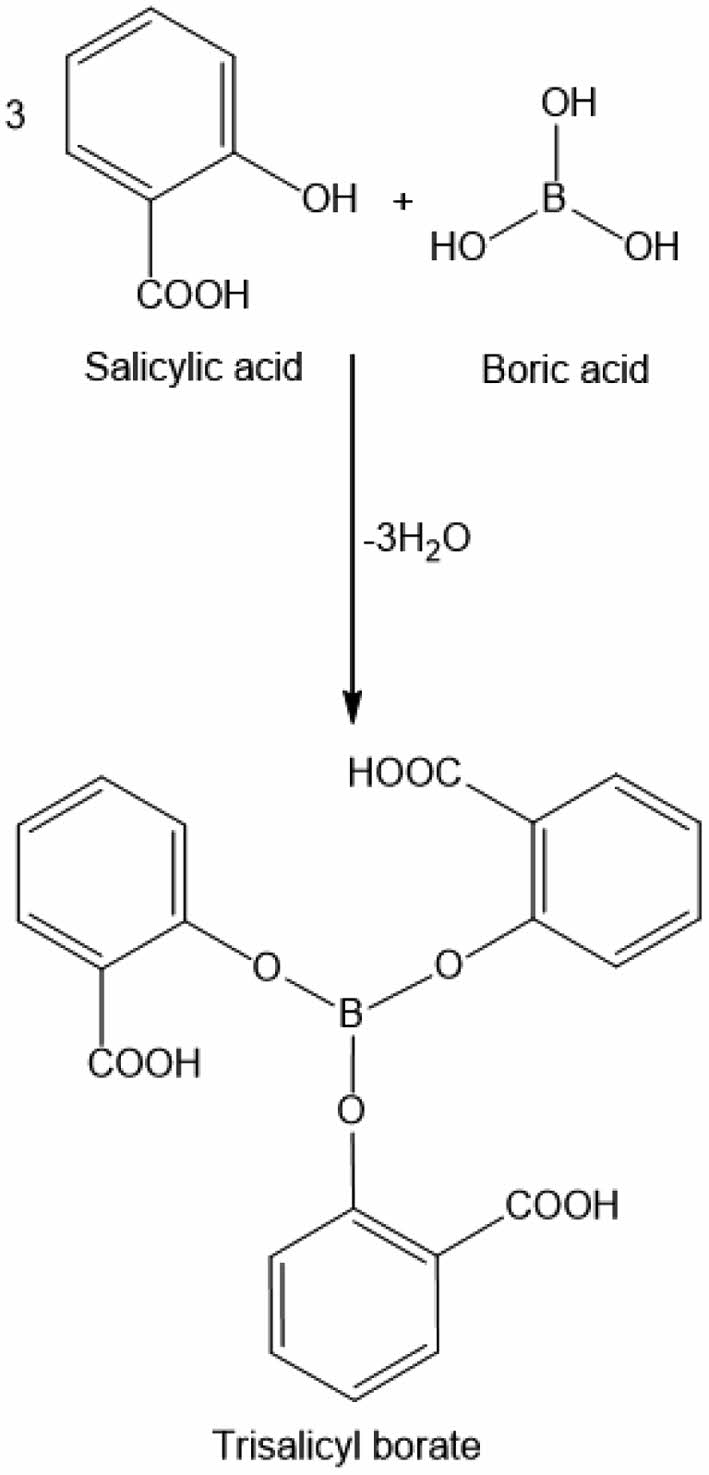
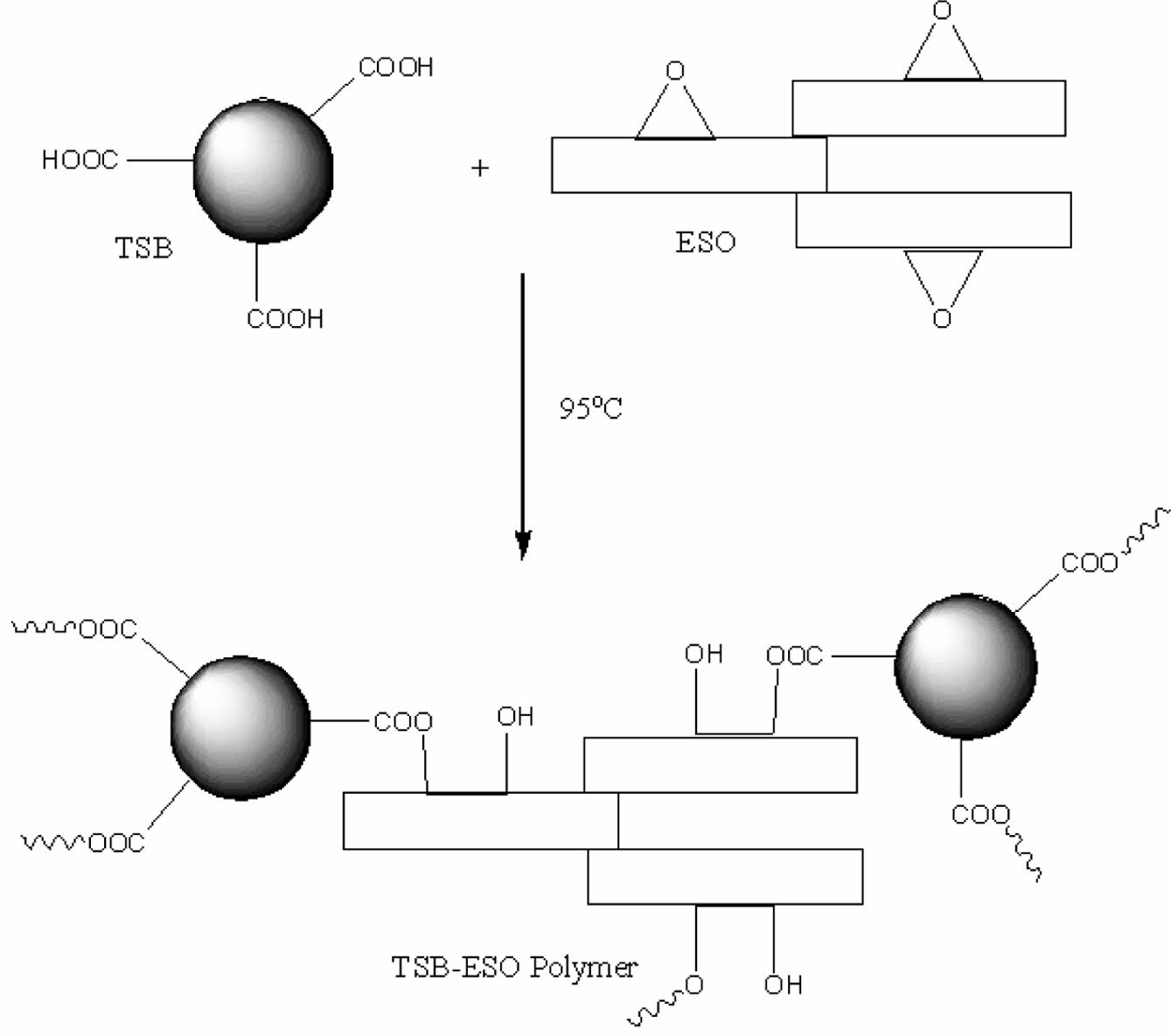
Among the many types of acids boric acid has some special features. When it dissolved in water the solution is not acidic. It is a weak acid that contains 3 hydroxyl groups. When boric acid is reacted with an aliphatic alcohol or a phenol, congruent ester is easily formed.16-18 Boric acid or boron compounds also impart the flame retardancy properties of the materials.19 Esterification reaction of boric acid and alcohols is generally carried under mild conditions. Those esterification reactions can just be monitored by measuring the amount of evaluated water. When boric acid reacted with a hydroxyl group containing carboxylic acid, carboxyl functionalized boric acid esters would be synthesized (Figure 1). Those carboxyl group containing boric acid ester also polymerizes with ESO through free carboxyl groups. Reaction proceeds abstraction of H+ ion by epoxide rings than nucleophilic attack of carboxylate group occurs. The final product is a hydroxyl group containing ester (Figure 2).
It is not possible to find a detailed study about polymers that are obtained from the reaction between ESO and carboxyl functionalized boric acid esters. This work investigates the synthesis and characterization of TSB-ESO as a novel polymeric material. Electrical properties of final rubbery materials are evaluated.
|
Figure 1 Synthesis and the structure of trisalicyl borate (TSB). |
|
Figure 2 Synthesis and the structure of TSB-ESO polymer. |
Materials and Methods. Epoxidized soybean oil (ESO) was kindly donated by Hall Star Company (Chicago, IL). Boric acid, diethyl ether, hexane, salicylic acid and toluene purchased from Merck (Darmstadt, Germany). Infrared (IR) characterization of compounds and polymers was performed by Nicolet 380 Fourier-transform infrared (FTIR) spectrometer with Smart Diamond ATR. The hydrogen nuclear magnetic resonance (1H NMR) spectra were recorded on a Varian 400-MHz NMR instrument operating at a frequency of 399.986 MHz for proton. Thermal stabilities of the material were characterized by “Thermal Analyses” Q 50 model thermogravimetric analysis (TGA) instrument with a 10 ℃/min heating rate under nitrogen atmosphere. Stress-Strain behavior was measured by Devotrans DVG 925 type instrument. Stress-strain test of the polymer was determined according to the ISO 8256 method with an elongation rate of 20 mm/min. Swelling ratios were measured with Gartner type travelling microscope. Electrical properties of the samples were tested by using GW-Instek LCR-6300 LCR Meter which supplies 2V AC voltage under varying (20 Hz to 2 kHz) frequencies.
Synthesis of TSB. In a 250 mL round bottom flask, 0.618 g (0.01 mol) boric acid and 4.15 g (0.03 mol) salicylic acid were mixed in 100 mL toluene. System was equipped with Dean-Stark apparatus and the mixture was refluxed until 0.5 mL (0.03 mol) water was collected. Then toluene was removed under reduced pressure. Trisalicyl borate was obtained as a white powder. TSB is a water sensitive material and should be stored in a desiccator.
Polymerization of ESO with TSB. For a typical polymerization reaction, 9.52 g (0.01 mol) of ESO was mixed with 4.22 g (0.01 mol) of TSB in a 50 mL beaker. After that, the mixture was transferred to a silicon mold quickly. The mold was then placed in a vacuum oven. Temperature was set to 95 ℃ and then the mixture was cured under nitrogen atmosphere. Reaction was completed in 6 hours.
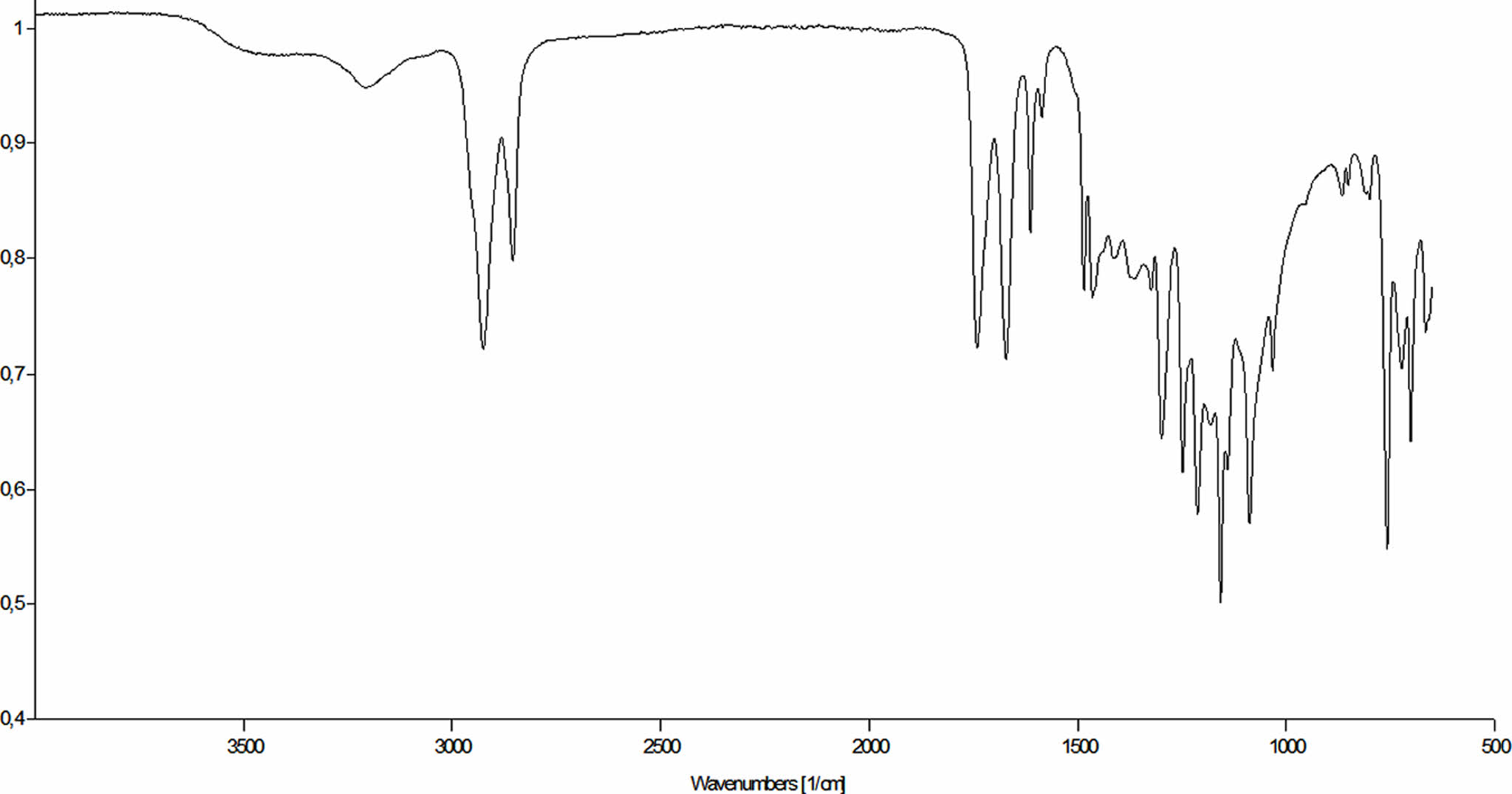
FTIR and NMR Characterization of Synthesized Materials. FTIR is a technique used to analyze the molecular composition of a sample by measuring the absorption or transmission of infrared radiation through the sample. The resulting spectrum can provide information about the functional groups and chemical bonds present in the sample.
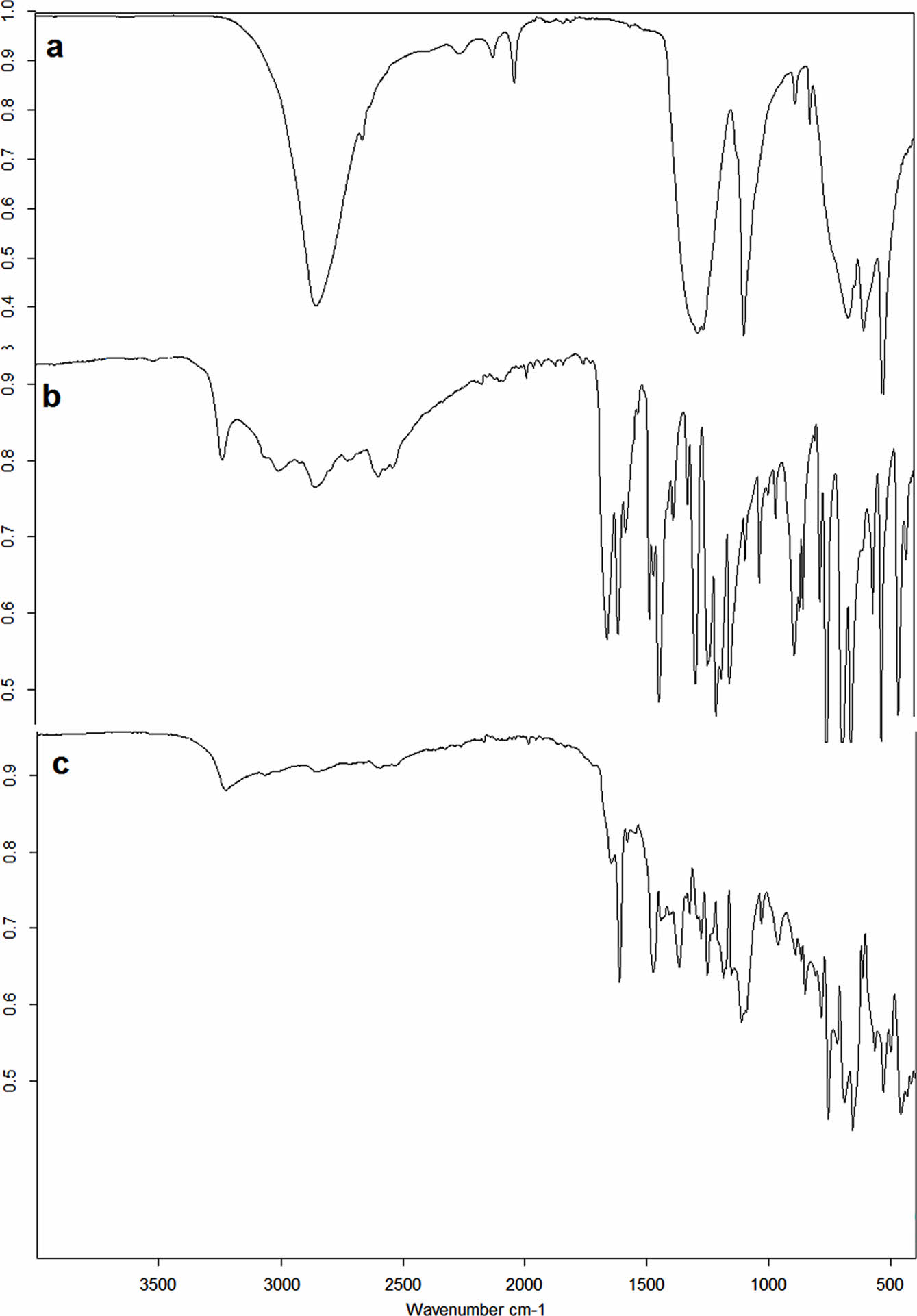
FTIR spectra of the starting materials and ESO-TSB polymer are shown in the Figure 2 and 3. The main peaks of the boric acid appeared at 3213, 1461, and 1194 cm-1. Characteristic peaks of salicylic acid, on the other hand, appeared at 3240, 3013, 1662, 1613, 1464, 1251, 1212, 1157, 760, 699, and 661 cm-1 respectively. When salicylic acid was esterified, trisalicyl borate was obtained. Characteristic peaks for TSB was observed at 3220, 1644, 1610, 1578, 1472, 1276, 784, 757, 656, and 530 cm-1. Esterification reactions were performed by using toluene azeotrope. At the temperatures toluene boils boric acid just reacted with free -OH groups of salicylic acid. The side product water, removed by toluene azeotrope. The peak of the boric acid at 3213 cm-1 and the peak of salicylic acid at 3240 cm-1 disappeared. A new peak observed at 3220 cm-1. Probably due to the interaction with free carboxyl groups and boron ester, the peak shifted to 1644 cm-1 and the intensity of the peak at 1610 cm-1 increased.
When TSB mixed with ESO, Polymerization reaction was completed in 6 hours. Characteristic peaks of TSB-ESO polymer appeared at 3209, 2925, 2855, 1741, 1672, 1613, 1585, 1212, 1157, 1087, and 757 cm-1.
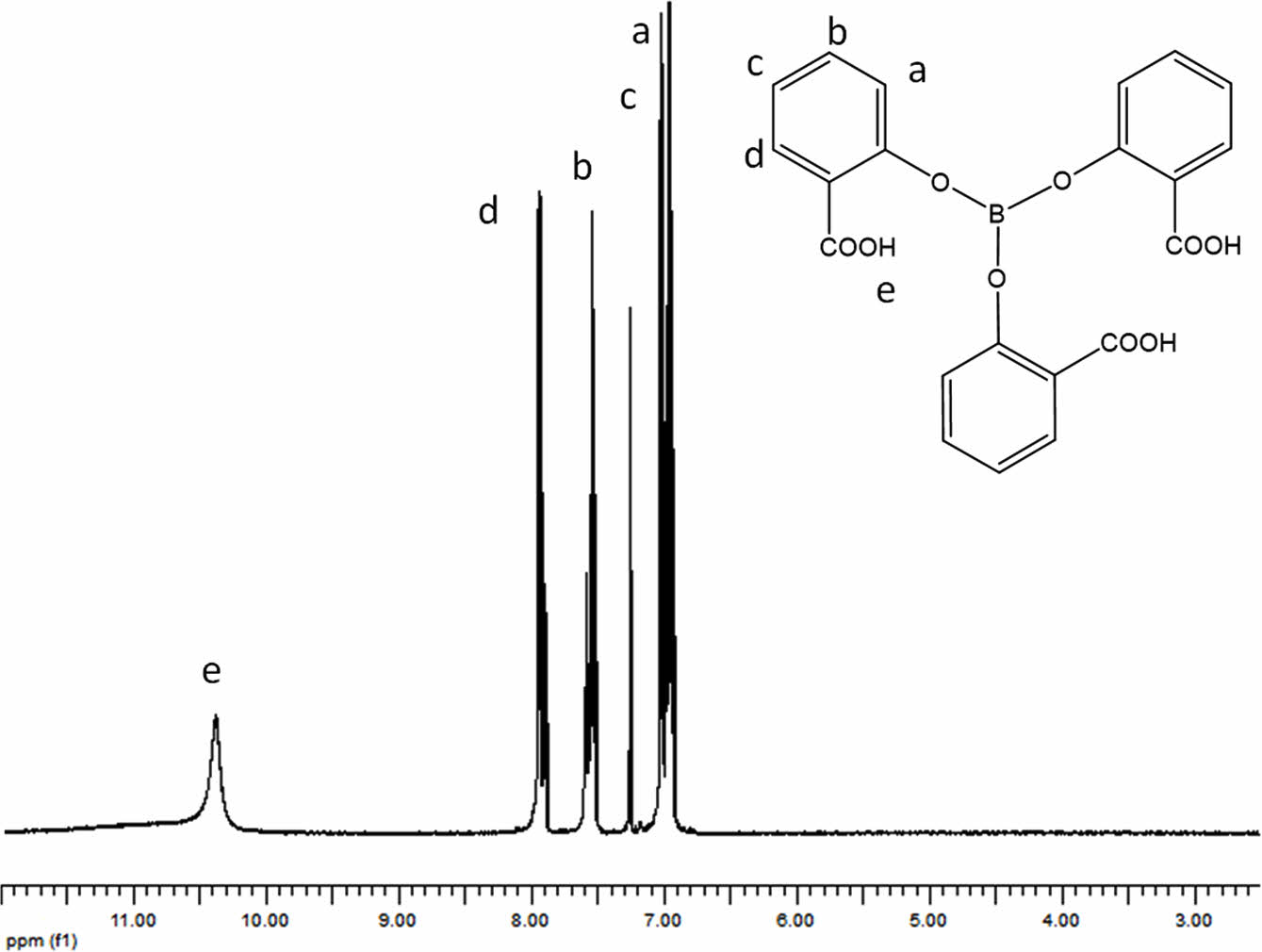
1H NMR spectrum of TSB is shown in the Figure 4. According to that spectrum, peaks revealed at 7.0, 7.6, 8.0, and 10.4 ppm. The peak at 7.0, 7.6, and 8.0 ppm depicted the presence of aromatic hydrogens. The integration ratio of those peaks was determined as 2/1/1 respectively. The peak at 10.4 ppm indicated the presence the hydrogen of free carboxyl group. Integration ratio of the hydrogens of carboxyl groups to aromatic hydrogens was determined as ¼.
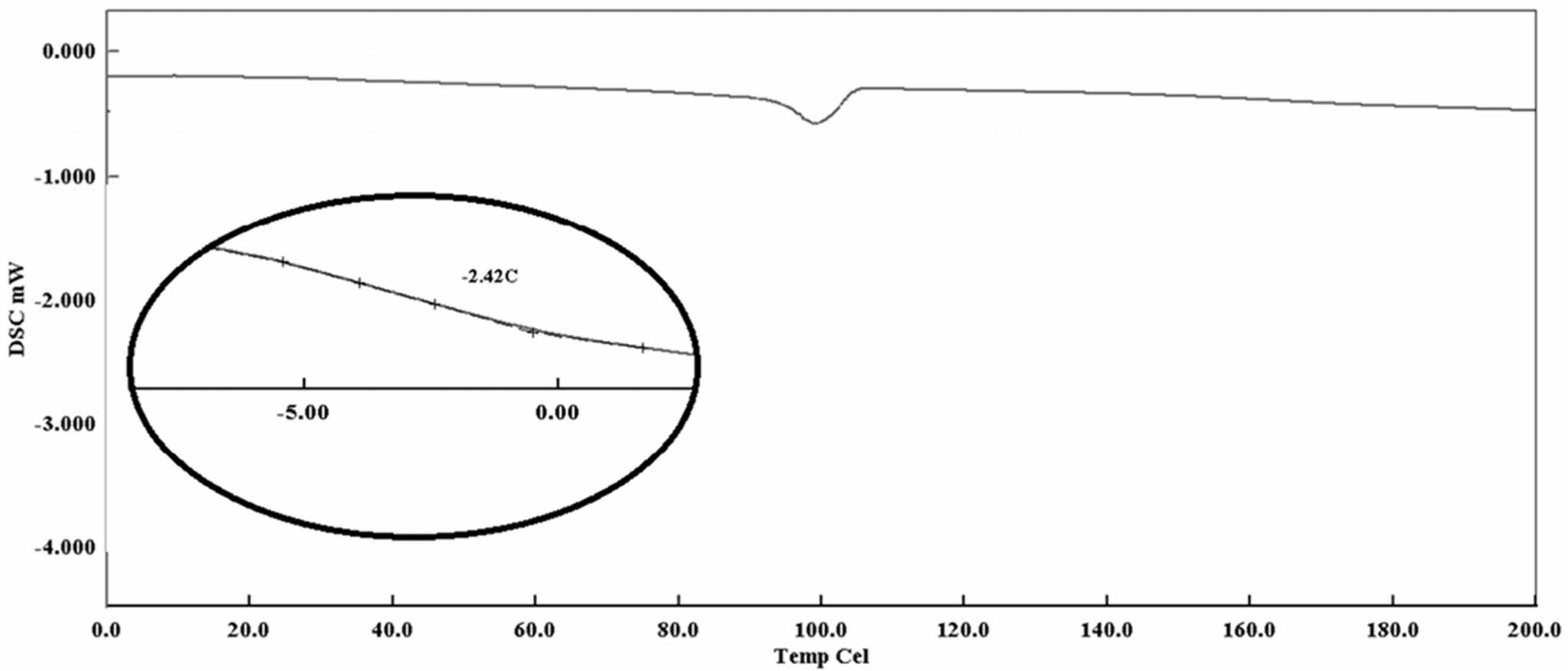
Thermal Characterization of Polymers. Differential scanning calorimetry (DSC) and TGA techniques were used to evaluate the thermal properties of the materials synthesized. DSC is a technique used to measure the thermal behavior of materials. It measures the difference in heat flow between a sample and a reference material as a function of temperature or time, while both materials are subjected to a controlled temperature program. DSC mainly used to determine the thermal stability, melting point, glass transition temperature. DSC graph of the TSB-ESO polymer is exhibited in Figure 5. According to that trace, the polymer exhibited a Tg at -2 ℃ and a melting point at 92 ℃. That melting behavior is typical for boric acid esters.20
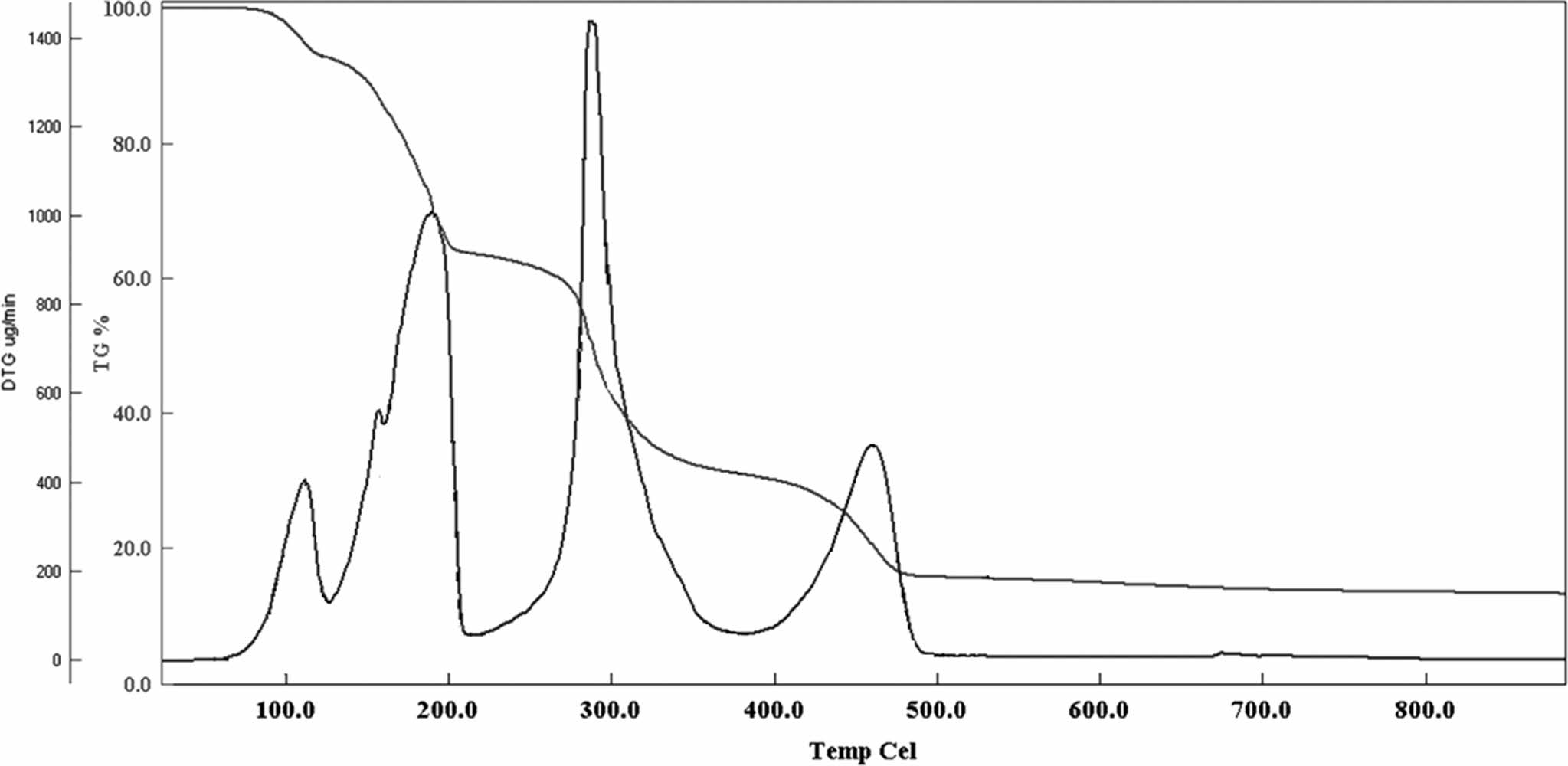
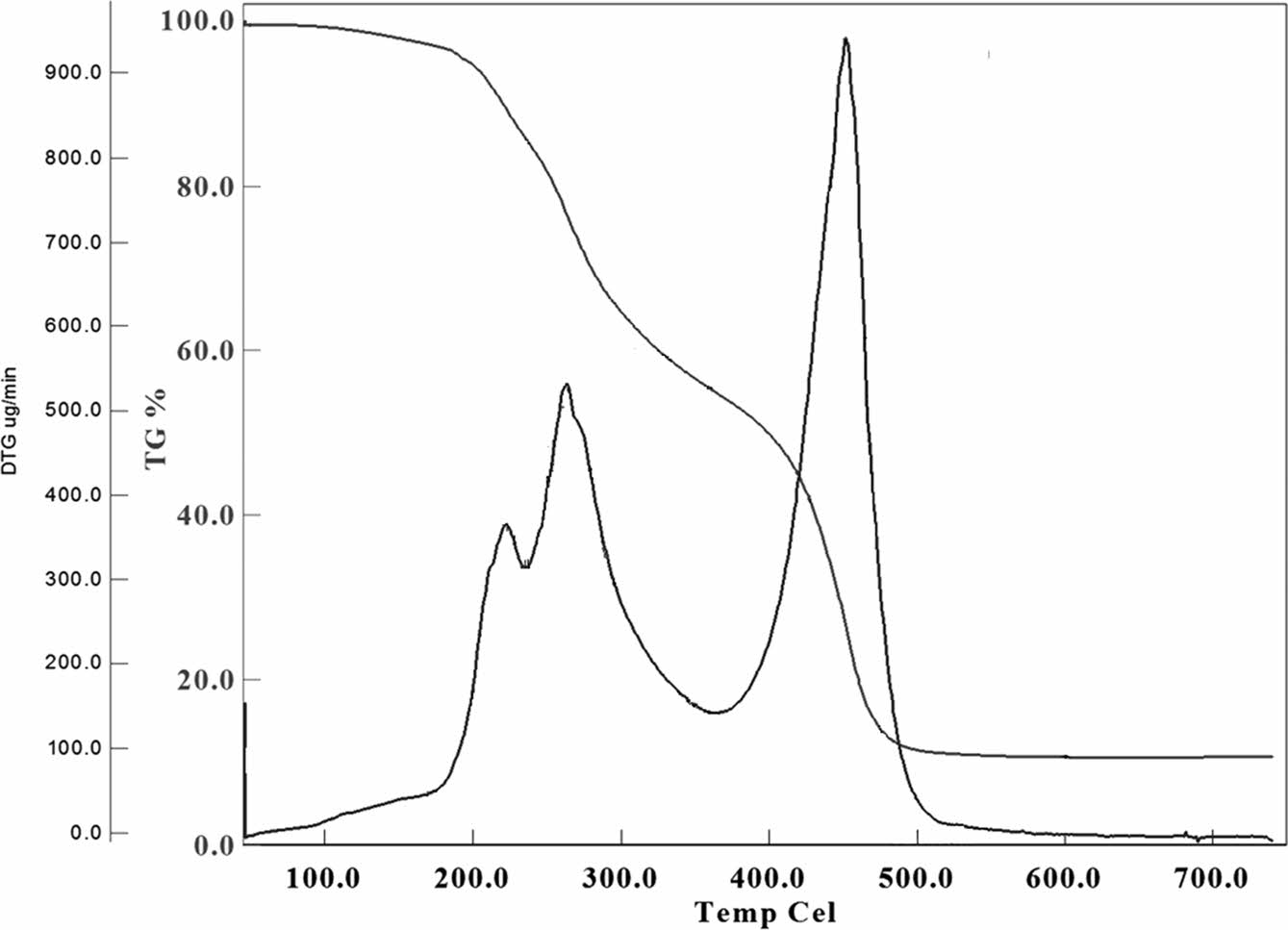
TGA is a technique used to study the thermal stabilities of materials by measuring the weight change as a function of temperature or time. This technique is widely used in order to elucidate the thermal stability, decomposition, and oxidation of materials. TGA graph of the TSB and TSB-ESO polymer are shown in Figure 6 and 7 respectively.
TGA thermogram revealed that during thermal decomposition, 4 plateau observed. First one started at 84 ℃ and completed at 121 ℃. Second decomposition started at 121 ℃ and ended at 206 ℃. Third one was observed between 206 and 375 ℃ and the last one revealed between 375 and 485 ℃. For each decomposition intervals 7.4, 29, 32.5, and 15.7% weight losses were observed respectively.
For polymers two types of information can be obtained from TGA measurements. Those are 5% and 50% weight loss temperatures. 5% weight loss temperature was identified as 185 ℃ and 50% weight loss was depicted as 405 ℃.
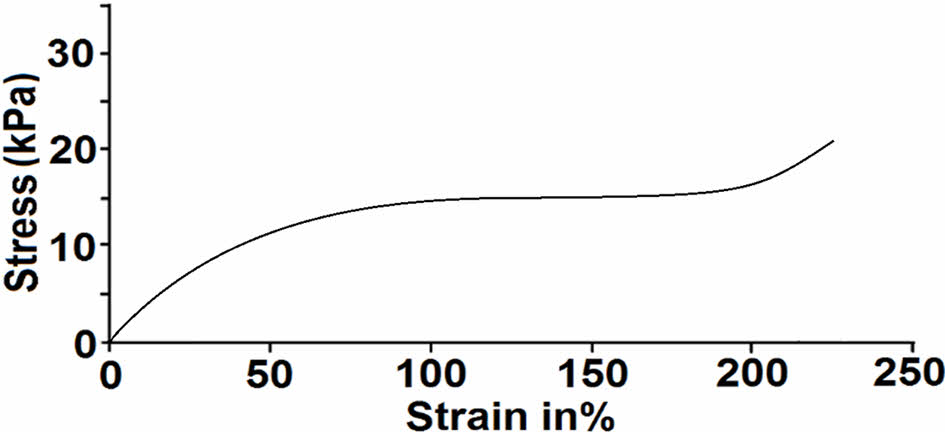
Mechanical Characterization of Polymers. In order to evaluate mechanical properties of the materials, stress-strain and swelling tests were applied. This test involves subjecting a sample of the material to a gradually increasing tensile or compressive force, while simultaneously measuring the resulting deformation or strain. The stress-strain curve obtained from this test provides valuable information about the material’s mechanical behavior, including its strength, stiffness, ductility, and toughness (Figure 8). Stress-strain test of the polymer was determined according to the ISO 8256 method with an elongation rate of 20 mm/min. TSB-ESO polymers showed high elongation with small stress values. Stress at break value was detected as 100 kPa with 230% elongation. Due to the lack of long chains.
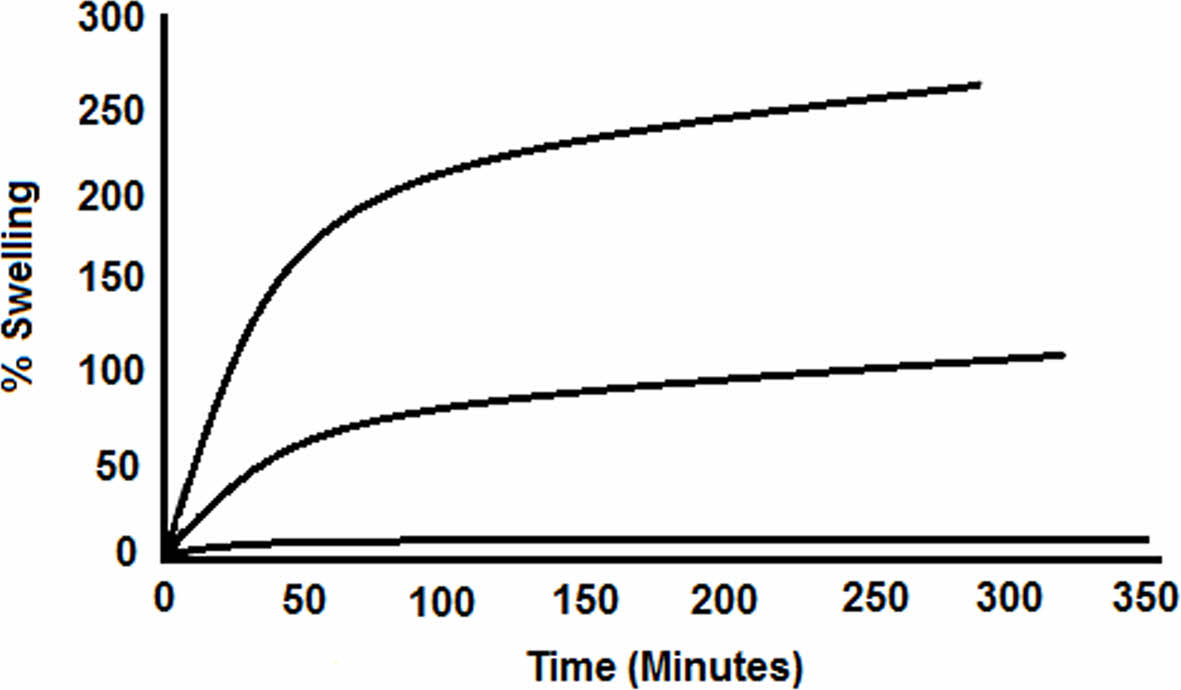
The swelling behaviors of the products in hexane, diethyl ether and toluene were evaluated by a traveling microscope.The samples were put in a closed container, and the experiment was continued until the solvent uptake ceased. Hexane, diethyl ether and toluene was used for trials (Figure 9). The highest % of swelling was measured in toluene the lowest value was determined in hexane case.
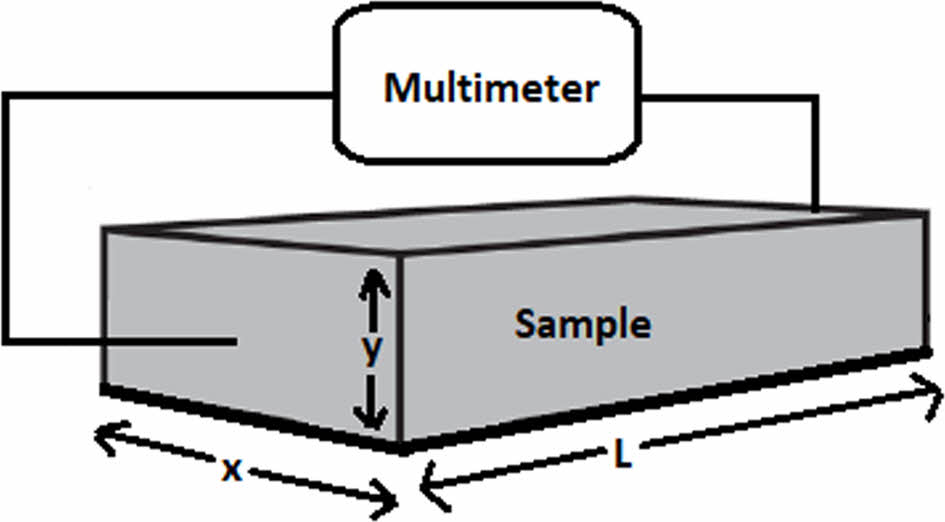
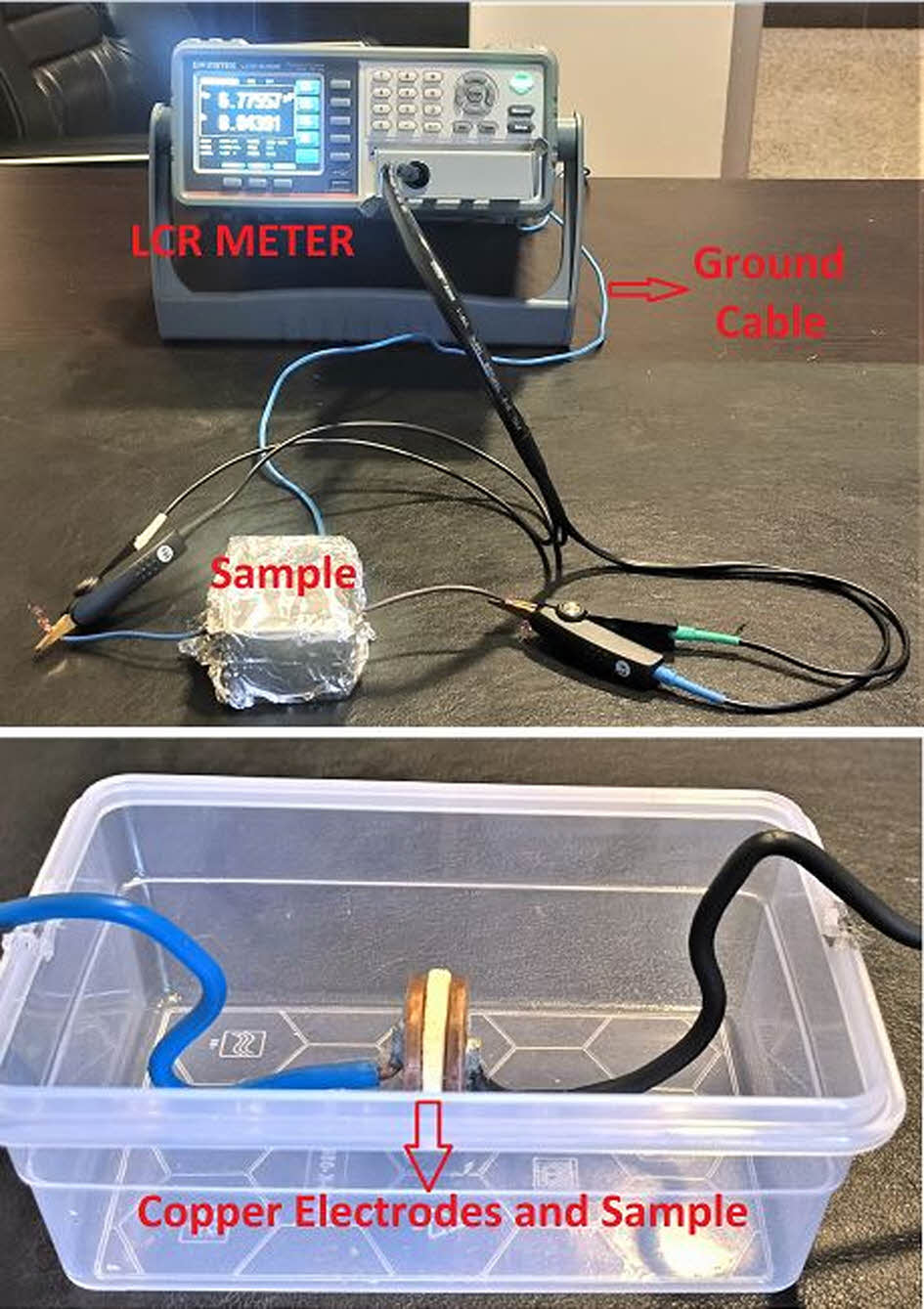
Electrical Properties of Polymers. Polymeric sample was tested based on its electrical properties under AC and DC supply voltages. In DC analysis,the polymeric sample’s resistivity and conductivity is measured by using two-point measurement technique.21-22 Two-point resistivity measurement is simple technique, which is rely on ohm’s law. The test set-up of this technique is given in Figure 10. In the test set-up the polymeric samples (x,y and L dimensions are known) are tested by using Unit UT502A Insulation Tester. During AC analysis polymeric sample is subjected to AC source with different frequency values. Circular samples (3 mm thickness) are placed between circular copper electrodes with the diameter of 2.89 cm. Copper electrodes are preferred due to their low resistance and high contact ability with sample. Samples are shielded and grounded to avoid electromagnetic interactions, which may interfere capacitance measurements. Samples are tested by using GW-Instek LCR-6300 LCR Meter which supplies 2V AC voltage under varying (20 Hz to 2 kHz) frequencies. Test set-up and the sample arrangement with copper electrodes are given in Figure 11 and Figure 12.
Polymeric sample exhibits insulator properties. DC analysis reveals that the resistivity and conductivity of the polymeric sample is measured as 2.375 MΩm and 0.401 µS/m respectively. According to AC analysis, sample is highly capacitive (it varies from 6.77 to 5.97 pF). With the increasing frequency, the impedance value of the sample is decreased (from about 1 GΩ to 10 MΩ). Based on its electrical properties, produced polymeric sample can be evaluated as insulator.
|
Figure 3 FTIR spectra of a- boric acid, b- salicylic acid and c- trisalicyl borate. |
|
Figure 4 FTIR spectrum of ESO-TSB polymer. |
|
Figure 5 1H NMR of trisalicyl borate. |
|
Figure 6 DSC trace of ESO-TSB polymer. |
|
Figure 7 TGA thermogram of TSB. |
|
Figure 8 TGA graph of ESO-TSB polymer. |
|
Figure 9 Stress-Strain curve of ESO-TSB polymer. |
|
Figure 10 Swelling Behaviors of ESO-TSB polymer. |
|
Figure 11 Two-point resistivity measurement set-up |
|
Figure 12 Impedance measurement set-up. |
The creation of alternative sources is crucial for sustainability and for environment. As an alternative material, the thermal, mechanical and electrical properties of the polymer of trisalicyl borate and epoxidized soybean oil is explained. Like a rubbery material, this polymer show low tensile strength with high strain. This polymer have good insulation properties and it would be used for electrical applications where high tensile strength is not needed such as transformer insulator or wire insulator.
- 1. Kahraman, C.; Orobello, C.; Cirella, G. T. Changing Dynamics with COVID-19: Future Outlook. In Human Settlements. Advances in 21st Century Human Settlements, Cirella, G.T., Ed.; Springer: Singapore, 2021; pp 235-252.
-

- 2. Lam, D. How the World Survived the Population Bomb: Lessons From 50 Years of Extraordinary Demographic History. Demography 2011, 48, 1231-1262.
-

- 3. Cayli, G.; Kusefoglu, S. Isothiocyanates of Plant Oil Triglycerides: Synthesis, Characterization and Polymerization with Polyols and Polyamines. J. Appl. Polym. Sci. 2010, 116, 125-131.
-

- 4. Park, S. J.; Jin, F. L.; Lee, J. R. Thermal and Mechanical Properties of Tetrafunctinal Epoxy Resin Toughened with Epoxidized Soybean Oil. Mater. Sci. Eng. A 2004, 374, 109-114.
-

- 5. Meier, M. A. R. Merhathesis with Oleochemicals: New Approaches for the Utilization of Plant Oils as Renewable Resources in Polymer Science. Macromol. Chem. Physic. 2019, 210, 1073-1079.
-

- 6. Cayli, G.; Kusefoglu, S. Polymerization of Linseed oil with Phenolic Resins. J. Appl. Polym. Sci. 2010, 118, 849-856.
- 7. Adhavaryu, A.; Erhan, S. Z. Epoxidized Soybean Oil as a Potential Source of High Temperature Lubricants. Ind. Crop. Prod. 2002, 15, 247-254.
-

- 8. Ma, S.; Webster, D. C. Naturally Occurring Acids as Cross-Linkers to Yield VOC-Free, High Performance, Fully Bio-Based, Degradable Thermosets. Macromolecules 2015, 48, 7127-7137.
-

- 9. Silva, J. A. C.; Grilo, L. M.; Gandini, A.; Lacerda, T. M. The Prospering of Macromolecular Materials Based on Plant Oils Within the Blooming Field of Polymers from Renewable Resources. Polymers 2021, 13, 1722.
-

- 10. Khot, S. N.; Lascala, J. J.; Can, E.; Morye, S. S.; Williams, G. I.; Palmese, G. R.; Kusefoglu, S. H.; Wool, R. P. Development and Application of Triglyceride-based Polymers and Composites. J. Appl. Polym. Sci. 2001, 82, 703-723.
-

- 11. Crivello, J. V.; Narayan, R. Epoxidized Triglycerides as Renewable Monomers in Photoinitiated Cationic Polymerization. Chem. Mater. 1992, 4, 692-699.
-

- 12. Cayli, G.; Kusefoglu, S. Polymerization of Acrylated Epoxidized Soybean Oil with Phenol Furfural Resins via Repeated forward and Retro Diels-Alder Reactions. J. Appl. Polym. Sci. 2011, 120, 1707-1712.
-

- 13. Zeng, R. T.; Wu, Y.; Li, Y. D.; Wang, M.; Zeng, J. B. Curing Behavior of Epoxidized Soybean Oil with Biobased Dicarboxylic Acids, Polym. Test. 2017, 57, 281-287.
-

- 14. Yang, J.; Ching, Y. C.; Ching, K. Y.; Ran, X.; Al-Hada, N. M.; Sui, X.; Wei, Y.; Yu, J.; Wang, J.; Zhou, J. Preparation and Characterization of Starch-Based Bioplastic Films Modified by Citric Acid-Epoxidized Soybean Oil Oligomers. J. Polym. Environ. 2023, 31, 954-964.
-

- 15. Doğan, E.; Küsefoğlu, S. Synthesis and In Situ Foaming of Biodegradable Malonic Acid ESO Polymers. J. Appl. Polym. Sci. 2008, 110, 1129-1135.
-

- 16. Alemdar, N.; Tuncer Erciyes, A. T.; Bicak, N. Preparation of Unsaturated Polyesters Using Boric Acid as Mild Catalyst and Their Sulfonated Derivatives as New Family of Degradable Polymer Surfactants, Polymer 2010, 51, 5044-5050.
-

- 17. Brown, H. C.; Mead, E. J.; Shoaf, C. J. Convenient Procedures for the Preparation of Alkyl Borate Esters. J. Am. Chem. Soc. 1956 78, 3613-3614.
-

- 18. Steinberg, H.; Hunter, D. L. Preparation and Rate of Hydrolysis of Boric Acid Esters. Ind. Eng. Chem. 1957, 49, 174-181.
-

- 19. Demirhan, Y.; Yurtseven, R.; Usta, N. The Effect of Boric Acid on Flame Retardancy of Intumescent Flame Retardant Polypropylene Composites Including Nanoclay. J. Thermoplastic Compos. Mater. 2023, 36, 1187-1214.
-

- 20. Houston, T. A.; Wilkinson, B. L.; Blanchfield, J. T. Boric Acid Catalyzed Chemoselective Esterification of α-Hydroxycarboxylic Acids. Org. Lett. 2004, 6, 679-681.
-

- 21. Sahebi, H. Z. Lashgari, V. A.; Mohammadi, M. H. D.; Uner, D.; Pourabdouli, M. Microstructure, Resistivity, and Shear Strength of Electrically Conductive Adhesives Made of Silver-coated Copper Powder. Microelectron. Reliab. 2021, 127, 114400.
-

- 22. Suryanto, B.; Takaoka, H.; McCater, W. J.; Saraireh, D.; Taha, H. Impedance Measurements on An Engineered Cementitious Composite: A Critical Evaluation of Testing Protocols. Measurement 2018, 129, 445-456.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2022 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2023; 47(5): 606-612
Published online Sep 25, 2023
- 10.7317/pk.2023.47.5.606
- Received on May 5, 2023
- Revised on Jun 19, 2023
- Accepted on Jun 26, 2023
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
- Conflict of Interest
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Gökhan Çayli
-
Istanbul University-Cerrahpaşa, Engineering Faculty, Department of Engineering Sciences, Istanbul 34320, Turkey
- E-mail: gokhan.cayli@iuc.edu.tr
- ORCID:
0000-0002-3395-5642









 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.