- Calculating Activation Energy of Phthalonitrile Using the Flynn-Wall-Ozawa Equation
Joon Hyuk Lee†
 , Eunkyung Jeon, Jung-kun Song, and Jaeho Choi
, Eunkyung Jeon, Jung-kun Song, and Jaeho ChoiAgency for Defense Development, Yuseong P.O. Box 35, Daejeon 34186, Korea
- Flynn-Wall-Ozawa 수식에 기반한 Phthalonitrile의 활성화 에너지 연구
국방과학연구소
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
This study employed the Flynn-Wall-Ozawa method to determine the activation energy (Ea) of phthalonitrile (PN) synthesized with varying concentrations of a sulfur-containing curing agent. The synthesis process, characterized by various routes differing in curing times and temperatures, revealed that an optimal balance is critical for ideal thermal characteristics. It was observed that a 15% concentration of the curing agent offered the highest thermal stability. However, the proportion of sulfur content did not directly correlate with the applied proportion of curing agents, hinting at uneven mixing during the process. Despite this, the experimental results underscore the importance of fine-tuning the curing parameters for optimal synthetic outcomes. Lastly, an elevated Ea was in a direct correlation with the dosage amount of curing agents which confirmed their enhanced thermal stability.
이 연구에서는 Flynn-Wall-Ozawa 방법을 사용하여 다양한 농도의 황 함유 경화제로 합성된 프탈로니트릴의 활성화 에너지(Ea)를 분석하였다. 경화 시간과 온도의 변화를 통해 최적 합성루트를 우선 수행하였으며, 이를 기반으로 5-15%의 경화제를 적용하여 제조된 샘플의 열특성을 분석하였다. 다양한 농도 중, 15% 농도의 경화제가 가장 높은 열 안정성을 나타내었다. 그러나, 황 함유량의 비율이 높아질수록 열 안정성의 증가폭은 점차 감소되는 것 또한 관찰되었다. 마지막으로, 샘플의 열 안정성과 비례상관적인 관계에 있는 Ea의 결과값을 통해 Flynn-Wall-Ozawa 수식의 유효성을 검증하였다.
Phthalonitrile was examined by altering the amount of sulfur-containing curing agent and utilizing different curing steps. Utilizing the Flynn-Wall-Ozawa equation, it was verified that the activation energy correlated with the thermal decomposition rate and the quantity of curing agent.

Keywords: heat-resistant material, phthalonitrile, curing agent, activation energy.
This research was funded by Agency for Defense Development, grant number 912989201.
The authors declare that there is no conflict of interest.
Understanding the activation energy (Ea) in heat-resistant materials is of utmost importance in material science.1 Ea serves as a fundamental parameter in characterizing the thermal stability of materials, where high Ea is often associated with enhanced thermal stability. The abovementioned provides a quantifiable measure of energy barrier that must overcome for a particular reaction to proceed. Given that, accurately determining Ea provides invaluable insights into the kinetic behavior of materials under thermal stress. Isothermal and non-isothermal methods represent the primary approaches used to determine the Ea. Isothermal methods require running a series of experiments at various temperatures, which is oftentimes time-consuming and resource-intensive. In contrast, non-isothermal methods have gained prominence for their comparative practicality and efficiency. Non-isothermal methods, involving controlled heating rate experiments, allow for the characterization of kinetic behavior over a range of temperatures in a single experiment. They use model-fitting or model-free methods to estimate the Ea. Model-fitting methods are restrictive as they presuppose the reaction mechanism, thus limiting their applicability.
Among non-isothermal model-free methods, the Kissinger, Ozawa, and Flynn-Wall-Ozawa (FWO) methods are widely explored.2-4 Kissinger method requires the determination of peak maxima in the differential thermal analysis, which can introduce potential errors. The Ozawa method overcomes the aforementioned limitation, yet introduces its own challenges including the accuracy of the approximation. Lastly, the FWO method is an improved iteration of the Ozawa method. The FWO method adopts the Dolye approximation, assuming a constant factor for the integral of the reaction model function. This method allows for calculating Ea without prior knowledge of the reaction mechanism, which is a significant advantage over other model-dependent methods. Furthermore, it enables reliable calculation of Ea without the need of the exact peak temperatures, thereby mitigating potential inaccuracies that may arise from experimental variations.5-6
Here, we employed the FWO method to evaluate the Ea of phthalonitrile (PN) synthesized using various proportions of the curing agent. PN is an aromatic compound that consists of a benzene ring bonded to two CN groups. This compound serves as a key component in the synthesis of heat-resistant polymers at high temperatures.7-9 PN is recognized for having superior heat resistance compared to epoxy, cyanate ether, benzoxazines, and even polyimides.10-11 The main drawback with PN is its cost. It is notably expensive and not easily obtainable. Thus, we optimized the synthesizing process and endeavored to enhance its heat resistance.
This work is constructed as followings. The Experimental section presents the schematic of the PN employed in the study. The synthetic process involves the variation of sulfur-containing curing agent, adjusted at concentrations of 5, 10, and 15%. Subsequent to the synthesis, samples underwent assessment for thermal properties. The raw data set amassed from the analyses were then utilized in the FWO method to compute the Ea.
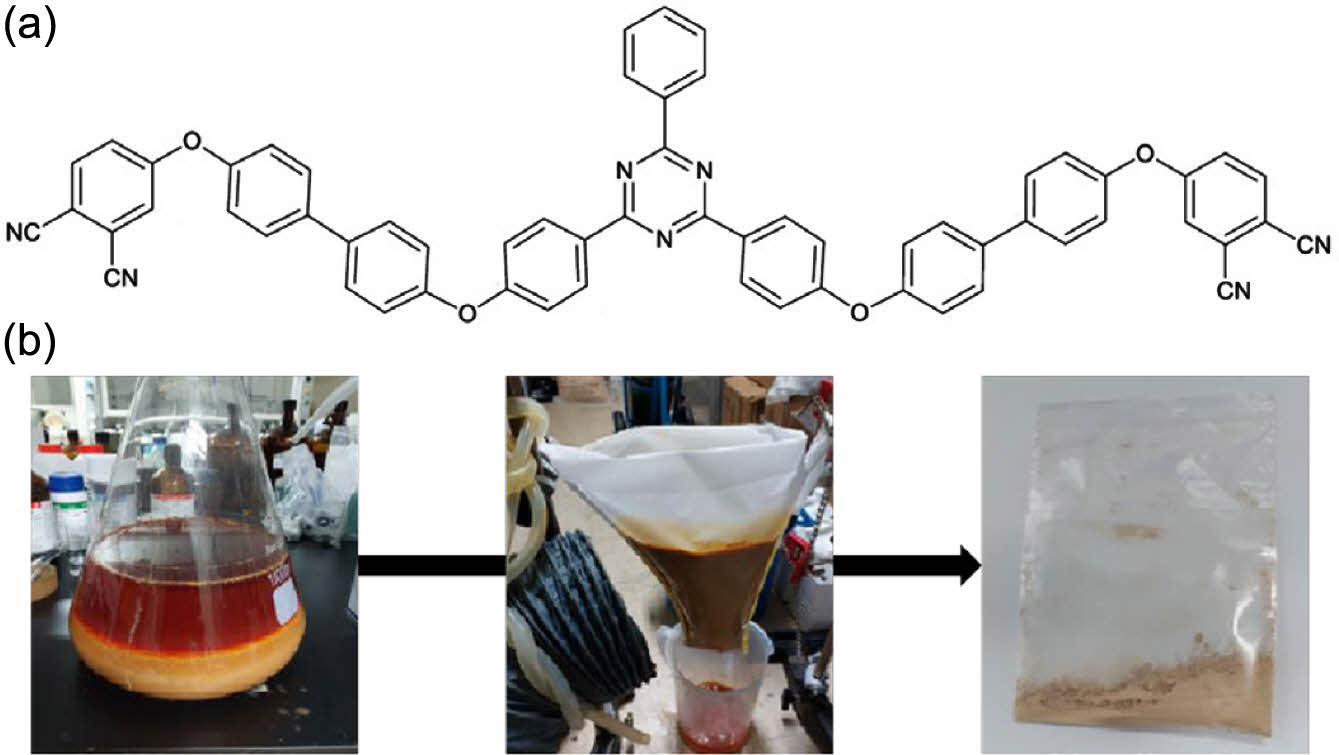
All chemicals were purchased from Sigma-Aldrich and used without purification. The initial mixture composed of 4-fluorobenzonitrile (200 g, 1.65 mol), benzoyl chloride (93 g, 0.66 mol), ammonium chloride (106 g, 1.98 mol), and aluminum chloride (106 g, 0.79 mol) was heated to 150 ℃ for 24 h. The subsequent cooling procedure entailed dropwise addition of cold water (1 L) until the temperature descended to 0 ℃, followed by the introduction of hydrochloric acid (36.5%, 200 g). The resultant compound 1 was subsequently washed with distilled water. The compound 2 was obtained by adding 4,4’-dihydroxybiphenyl (82 g, 0.44 mol), calcium carbonate (82 g, 0.58 mol), 1-methyl-2-pyrrolidone (600 mL), and toluene (600 mL) to compound 1, and heated to 150 ℃ (Figure 1). Toluene was removed via a Dean-Stark apparatus and the remaining solution cooled. The preceding compound (76 g, 0.22 mol) and 1-methyl-2-pyrrolidone (500 mL) were integrated at 150 ℃ for 3 h, followed by the addition of 4-nitrophthalonitrile (89 g, 0.51 mol) and 1-methyl-2-pyrrolidone (500 mL) at 50 ℃. The final compound was achieved via MeOH suspension and dried after the nonwoven filtration for three times. Verification of the surface characteristics was done using scanning electron microscopy (SEM) with energy dispersive X-ray (EDX) microanalysis (FEI Quanta 650 FEG SEM, Thermofisher Scientific). Owing to a high glass transition approaching 400 ℃, differential scanning calorimetry results were omitted due to the absence of a significant peak. Thermal properties were assessed via time-domain thermogravimetric using TGA-8000 (PerkinElmer).
|
Figure 1 Designated curing stages varied by temperature and time frame.Compound 2 was obtained as depicted in (a) by the following; (b) MeOH suspension and drying process via the nonwoven fabric filtration for three times. |
The curing process proves to be indispensable in the context of PN synthesis.12-13 It transitions the as-synthesized polymer from a state of low molecular weight and linear conformation to a high molecular weight configuration that exhibits a 3D network structure. This pivotal transformation owes its occurrence to the formation of crosslinks between polymer chains. In this work, curing agents with sulfur constituents were used for further applications.14
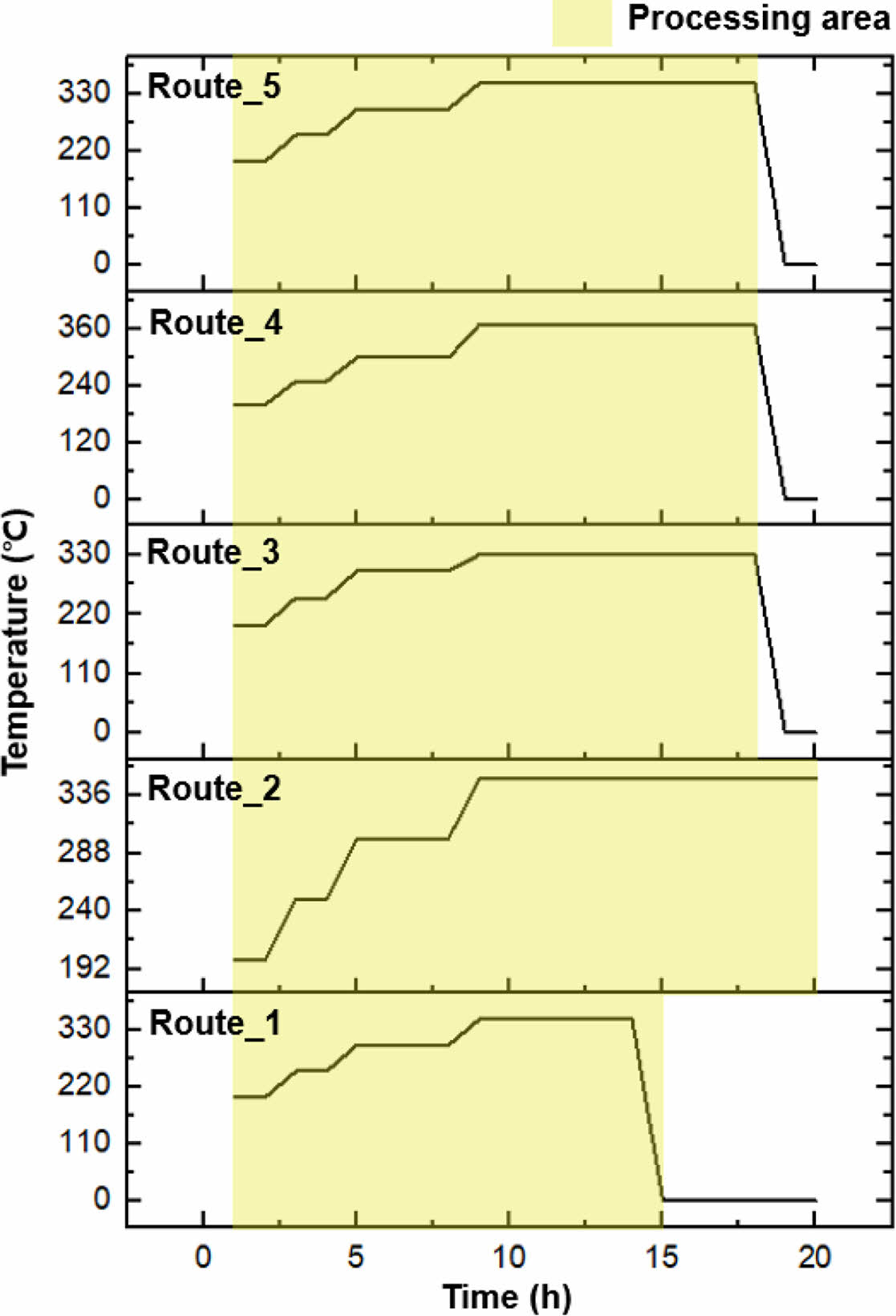
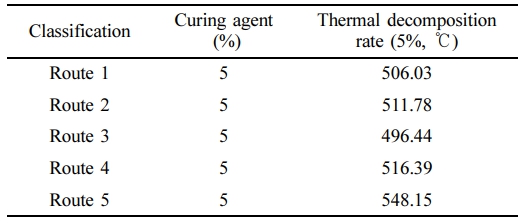
Our work encompassed various stages to assess the effects of varied curing times and temperatures (Figure 2 and Table 1). Each stage utilized a constant 5% sulfur-containing curing agent. Routes 1 and 2 are characterized by the shortest and longest final curing times, respectively. The brief curing duration in Route 1 insufficiently facilitated the monomer-to-polymer conversion, while in Route 2, an overlong curing duration risked polymer degradation. Routes 3 and 4, marked by the lowest and highest curing temperatures, respectively, also underperformed in terms of thermal endurance. While higher temperatures can expedite crosslinking reactions, an overly elevated temperature can induce premature crosslinking, thus resulting in an inhomogeneous network structure. Conversely, low temperatures as encountered in Route 3 may yield an inadequate curing process. It was observed that Route 5, which balances the extremes of both time and temperature, showcased superior thermal characteristics. Therefore, the findings underscore the importance of not only selecting an appropriate curing agent, but also optimizing the curing parameters to ensure optimal synthetic outcomes.
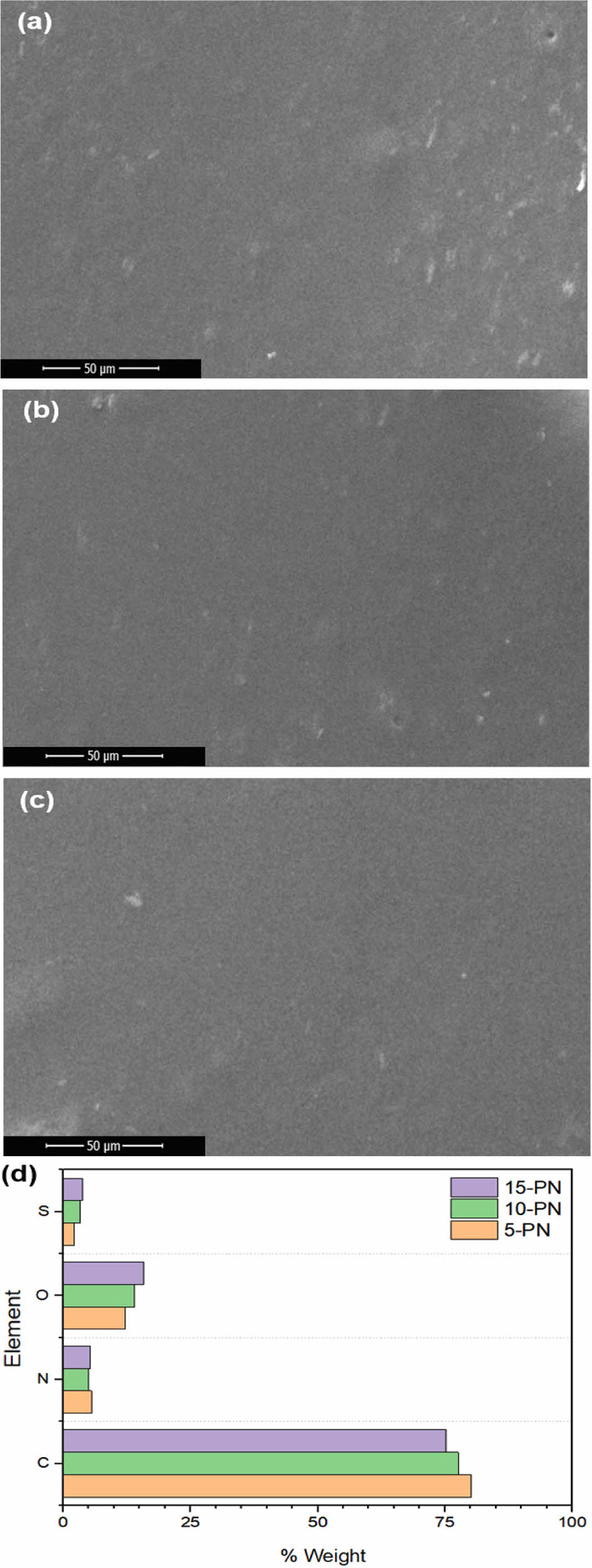
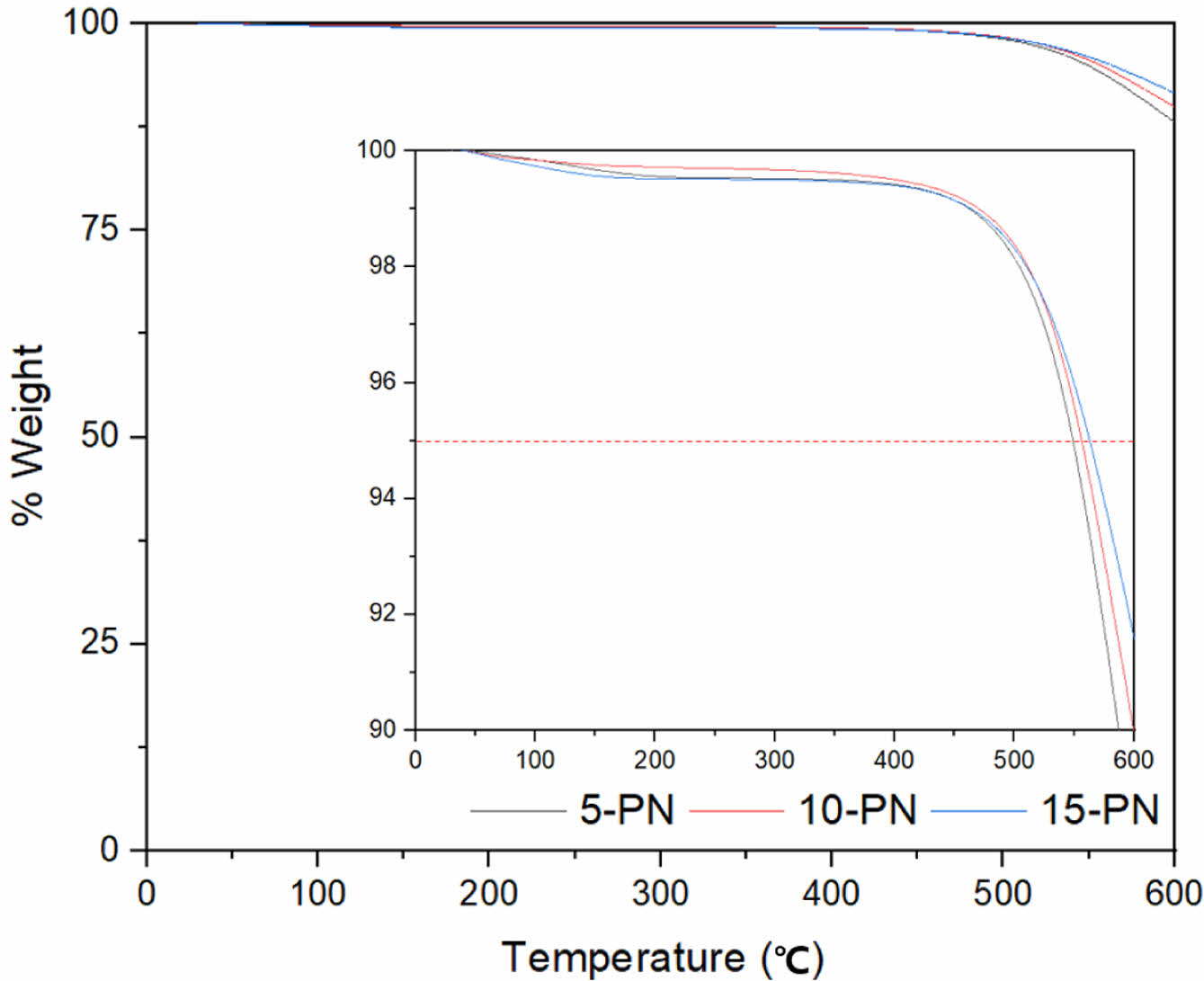
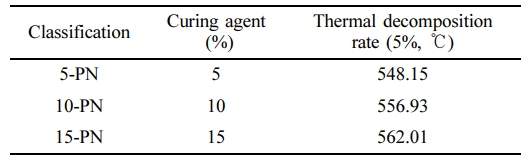
Regarding the aforementioned synthetic pathways, our work pursued Route 5, and further varied the proportion of the curing agent by 5, 10, and 15%. For the sake of simplicity, the derived samples were denoted as 5-PN, 10-PN, and 15-PN, respectively. Successful treatment of the curing on the PN was evidenced as displayed in Figure 3. Upon visual examination, SEM images of each sample did not reveal distinguishable differences. However, EDX results indicated more perceptible variations with different ratio of curing agents. It is noteworthy that the proportion of sulfur content does not linearly correlate with the applied proportion of curing agents. This deviation can be attributed to uneven mixing during the process, thus hindering a complete homogenization. Nevertheless, the sulfur content ratio was observed in a descending order from 15-PN, 10-PN, to 5-PN. TGA results demonstrated that the 15% of curing agent imparted the highest thermal stability, with a decrease evident as the proportion of curing agent was reduced (Figure 4 and Table 2). Interestingly, the difference between the thermal stability of samples with 10% and 15% curing agent was not as substantial as that between 5% and 10%. This could potentially be attributed to the inherent spatial constraints within the PN structure, which may limit the degree of interaction with the curing agent. In this part, further modelling studies are required to explore the realistic molecular behavior.
The calculated Ea values are shown in Table 3. Ea values indicate the energy barrier that must overcome for the reaction to occur, thereby crucial for understanding the thermal characteristics of samples. Samples with higher Ea values are less likely to decompose at lower temperatures and can resist high temperatures before starting to degrade. Speaking of PN, cross-linked PN in the early stages is expected to have high thermal stability due to the high density of strong covalent bonds. However, the weaker bonds in the polymer network will start to break down as the temperature increases. Upon further heating, the degradation of the main polymer backbone starts to occur.15-16 This is generally a multi-step process where the aromatic rings in the polymer backbone undergo various transformations including condensation reactions, oxidation and more. By reviewing the thermal decomposition rates of samples, it was confirmed that the FWO method appears to be an apt fit for evaluating thermal characteristics of PN.
|
Figure 2 Designated curing stages varied by temperature and time frame. |
|
Figure 3 SEM results of (a) 5-PN; (b) 10-PN; (c) 15-PN. Following EDX results are plotted in (d). |
|
Figure 4 TGA results of samples by various proportions of curing agent. Here, the 5% weight loss point is highlighted given that the aforementioned is a commonly reported metric for a comparison. |
|
Table 1 Thermal Profiles of Samples Obtained by Various Synthetic Routes at 5% of Decomposition Rate (air) |
|
Table 2 Thermal Profiles of Samples Obtained by Various Synthetic Routes at 5% of Decomposition Rate (air) |
This study implemented the FWO method to determine the Ea of PN synthesized using different amount of sulfur-containing curing agent. The best thermal properties were observed with a balanced curing time and temperature. Although SEM images of each sample appeared similar, EDX results displayed significant variations. The sulfur content ratio was observed to decrease in the order from 15-PN, 10-PN, to 5-PN. TGA results revealed that 15% curing agent provided the most thermal stability. Intriguingly, the disparity in thermal stability between the 10% and 15% samples was less evident than that between the 5% and 10% samples. This could be attributed to the sulfur ratio not aligning perfectly with the curing agent percentages, suggesting potential inherent spatial constraints within the PN structure. The calculated Ea values further substantiated the thermal resilience of the samples. As the temperature rises, the degradation process initiates with the breakdown of the weaker bonds, subsequently impacting the primary polymer backbone. Further investigations are essential to decode this molecular behavior in depth. The implications of this research could be far-reaching, offering insights for enhanced polymer synthesis, especially in applications demanding high thermal resistance.
- 1. Yanwu, Y.; Hui, M.; Jiahu, G.; Jingwei, M.; Suming, J.; Guimin, C.; Zihui, W. Thermal Analysis of a New Heat-Resistant Energetic Material. Sci. Adv. Mater. 2022, 14, 147-154.
-

- 2. Li, X.; Yao, H.; Lu, X.; Chen, C.; Cao, Y.; Xin, Z. Effect of Pyrogallol on the Ring-opening Polymerization and Curing Kinetics of a Fully Bio-based Benzoxazine. Thermochim. Acta 2020, 694, 178787.
-

- 3. Zheng, T.; Xi, H.; Wang, Z.; Zhang, X.; Wang, Y.; Qiao, Y.; Wang, X. The Curing Kinetics and Mechanical Properties of Epoxy Resin Composites Reinforced by PEEK Microparticles. Polym. Test. 2020, 91, 106781.
-

- 4. Zhao, X.; Huang, Z.; Song, P.; Yang, H.; Zhang, Y. Curing Kinetics and Mechanical Properties of Fast Curing Epoxy Resins with Isophorone Diamine and N-(3-aminopropyl)-imidazole. J. Appl. Polym. Sci. 2019, 136, 47950.
-

- 5. Miao, W.; Li, X.; Wang, Y.; Lv, Y. Pyrolysis Characteristics of Oil-field Sludge and the Comparison of Kinetic Analysis with Two Representative Methods. J. Pet. Sci. Eng. 2019, 182, 106309. J. Pet. Sci. Eng. 2019, 182, 106309. Eng 2019, 182, 106309.
-

- 6. Alashmawy, M. M.; Hassan, H. S.; Ookawara, S. A.; Elwardany, A. E. Thermal Decomposition Characteristics and Study of the Reaction Kinetics of Tea-waste. Biomass. Bioenergy 2023, 1-19.
-

- 7. Gu, H.; Gao, C.; Du, A.; Guo, Y.; Zhou, H.; Zhao, T.; Guo, Z. An Overview of High-performance Phthalonitrile Resins: Fabrication and Electronic Applications. J. Mater. Chem. C 2022, 10, 2925-2937.
-

- 8. Wu, M.; Xu, J.; Bai, S.; Chen, X.; Yu, X.; Naito, K.; Zhang, Q. A High-performance Functional Phthalonitrile Resin with a Low Melting Point and a Low Dielectric Constant. Soft Matter 2022, 16, 1888-1896.
-

- 9. Wang, Z.; Hu, J.; Zeng, K.; Yang, G. High Performance Phthalonitrile Cure Reaction and Property Research with a Novel Adenine-containing Aromatic Amine. Polym. Korea 2017, 41, 761-768.
-

- 10. Yakovlev, M. V.; Morozov, O. S.; Afanaseva, E. S.; Bulgakov, B. A.; Babkin, A. V.; Kepman, A. V. Tri-functional Phthalonitrile Monomer as Stiffness Increasing Additive for Easy Processable High Performance Resins. React. Funct. Polym. 2020, 146, 104409.
-

- 11. Poliakova, D. I.; Morozov, O. S.; Nechausov, S. S.; Afanaseva, E. A.; Bulgakov, B. A.; Babkin, A. V.; Avdeev, V. V. Fast Curing Phthalonitrile Modified Novolac Resin: Synthesis, Curing Study and Preparation of Carbon and Glass Fibric Composites. React. Funct. Polym. 2022, 181, 105450.
-

- 12. Kumar, D.; Choudhary, V. Curing Kinetics and Thermal Properties of Imide Containing Phthalonitrile Resin Using Aromatic Amines. Thermochim. Acta 2020, 693, 178749.
-

- 13. Chen, Z.; Wang, L.; Lin, J.; Du, L. A Theoretical Insight Into the Curing Mechanism of Phthalonitrile Resins Promoted by Aromatic Amines. Phts. Chem. Chem. Phys. 2021, 23, 17300-17309.
-

- 14. Zhang, X.; Wang, X., Li, J.; Zhang, S.; Zhang, Q.; Yu, X. Synthesis of Pyridine Heterocyclic Low-melting-point Phthalonitrile Monomer and the Effects of Different Curing Agents on Resin Properties. Polymers 2022, 14, 4700.
-

- 15. Park, Y.; Kang, H. J. The Physical Properties of Maleic Anhydride Polypropylene Film with Nano Cellulose Fiber. Polym. Korea 2020, 44, 201-207.
-

- 16. Lobanova, M. S.; Aleshkevich, V. V.; Yablokova, M. Y.; Morozov, O. S.; Babkin, A. V.; Kepman, A. V.; Bulgakov, B. A. Kinetics of the Oxidative Aging of Phthalonitrile Resins and Their Effects on the Mechanical Properties of Thermosets. Thermochim. Acta 2023, 724, 179492.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2022 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2024; 48(1): 41-45
Published online Jan 25, 2024
- 10.7317/pk.2024.48.1.41
- Received on Aug 21, 2023
- Revised on Sep 26, 2023
- Accepted on Nov 21, 2023
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
- Conflict of Interest
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Joon Hyuk Lee
-
Agency for Defense Development, Yuseong P.O. Box 35, Daejeon 34186, Korea
- E-mail: flower@hanyang.ac.kr
- ORCID:
0000-0002-0955-8743










 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.